- List of chemical compounds with unusual names
-
Chemical nomenclature, replete as it is with compounds with complex names, is a repository for some very peculiar and sometimes startling names. A browse through the Physical Constants of Organic Compounds in the CRC Handbook of Chemistry and Physics (a fundamental resource) will reveal not just the whimsical work of chemists, but the sometimes peculiar compound names that occur as the consequence of simple juxtaposition. Some names derive legitimately from their chemical makeup, from the geographic region where they may be found, the plant or animal species from which they are isolated or the name of the discoverer.
Some are given intentionally unusual trivial names based on their structure, a notable property or at the whim of those who first isolate them. However, many trivial names predate formal naming conventions. Trivial names can also be ambiguous or carry different meanings in different industries, geographic regions and languages.
Godly noted that "Trivial names having the status of INN or ISO are carefully tailor-made for their field of use and are internationally accepted".[1] In his preface to Chemical Nomenclature, Thurlow wrote that "Chemical names do not have to be deadly serious".[2] A classic website in existence since 1997[3] and maintained at the University of Bristol lists a selection of molecules with silly or unusual names strictly for entertainment. These so-called silly or funny trivial names (of course depending on culture) can also serve an educational purpose. In an article in the Journal of Chemical Education, Dennis Ryan argues that students of organic nomenclature (considered a dry and boring subject) may actually take an interest in it when tasked with the job of converting funny-sounding chemical trivial names to their proper systematic names.[4]
The collection listed below presents a sample of trivial names and gives an idea how chemists are inspired when they coin a brand new name for a chemical compound outside of systematic naming. It also includes some examples of systematic names and acronyms that accidentally resemble English words.
Contents
Elements
Glenn Seaborg proposed the chemical symbol Pu (from P.U.) for plutonium as a joke, only to find it officially adopted.[citation needed] Unununium (Uuu) was the former temporary name of the chemical element number 111, a synthetic transuranium element. This element was named roentgenium (Rg) in November 2004.
List of compounds
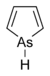
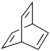
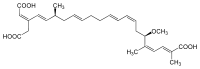
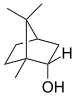
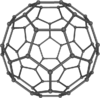
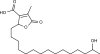
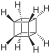
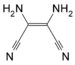
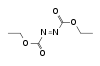
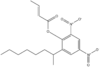
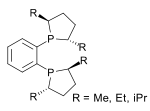
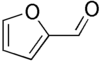
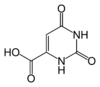
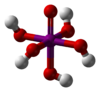
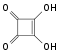
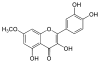
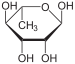
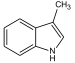
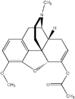
Adamantane (tricyclo[3.3.1.13,7]decane), a crystalline cycloalkane,[5][6] an isomer of twistane. Alcindoromycine an anthracycline antibiotic agent named after the character Alcindoro in La Bohème.[7] Angelic acid An organic acid found in garden angelica (Angelica archangelica), Umbelliferae, and many other plants. Arsole (C4H5As), an analogue of pyrrole in which an arsenic atom replaces the nitrogen atom.[8] The aromaticity of arsoles has been debated for many years.[9] The compound in which a benzene ring is fused to arsole — typically on the carbon atoms 3 and 4 — is known as benzarsole. BARF (tetrakis[3,5-bis(trifluoromethyl)phenyl]borate), a fluoroaryl borate B(Ar(CF3)2)4–, used as a non-coordinating anion Barrelene (C8H8), the name derives from the resemblance with a barrel.[10] Basketane pentacyclo[4.4.0.02,5.03,8.04,7]decane (C10H12), a polycyclic alkane with a structure similar to a basket. Bastardane a close relative to adamantane and its proper name is ethano-bridged noradamantane. Because its unusual ethano-bridge was a deviation from the standard hydrocarbon caged rearrangements, it came to be known as bastardane—the unwanted child.[11] Bohemamine an anti-tumour agent named after the Puccini opera La Bohème.[7] Bongkrek acid a deadly respiratory toxin named after the fermented coconut dish tempe bongkrèk in which it occurs after contamination with the bacterium Burkholderia cocovenenans. Its name resembles a combination of Bong, Crack and Acid. Borneol Named after the island Borneo. Buckminsterfullerene or buckyballs, a form of carbon named after Buckminster Fuller due to its resemblance to Fuller's geodesic domes. The term was coined by Harold Kroto.[12] The alternative name Footballene was coined by A.D.J. Haymet[13] because the molecule also resembles a football. Bullvalene tricyclo[3.3.2.02,8]deca-3,6,9-triene (C10H10), was named by organic chemist Maitland Jones, Jr. for William "Bull" Doering who predicted its properties in 1963.[14][15] Within a specific temperature range the molecule is subject to rapid degenerate Cope rearrangements with the result that all carbon atoms and hydrogen atoms are equivalent and that none of the carbon-carbon bonds is permanent. Cadaverine a foul-smelling diamine produced by putrefaction of dead animal tissue.[16] catP the name of the enzyme responsible for chloramphenicol resistance in various species of bacteria. Collinemycin an anthracycline antibiotic agent named after the character Colline in La Bohème.[7] Constipatic acid [2-(14-hydroxypentadecyl)-4-methyl-5-oxo-2,5-dihydrofuran-3-carboxylic acid], an aliphatic acid derived from the Australian Xanthoparmelia lichen.[17] Crapinon an anticholinergic drug, one side effect of which is constipation Cubane a hydrocarbon whose eight carbon atoms occupy the vertices of a cube.[18] Cumene The common name for isopropylbenzene. Cummingtonite ((Mg,Fe)7Si8(OH)22), a magnesium-iron silicate hydroxide, first identified in Cummington, Massachusetts. DAMN Diaminomaleonitrile, an organic chemical that contains two amine groups and two nitrile groups boud to an ethylene backbone. Diabolic acid a series of long-chain dicarboxylic acids with chains of different lengths. Named after the Greek word diabollo meaning to mislead.[19] DEAD an apt acronym, given that diethyl azodicarboxylate is explosive; shock sensitive; carcinogenic; and an eye, skin, and respiratory irritant. Dickite (Al2Si2O5(OH)4), a clay-like material with a number of manufacturing uses, one of which is as a coating for high-quality bond paper. It is named after its discoverer, Dr. W. Thomas Dick. Dinocap (C18H24N2O6), a miticide and contact fungicide used to control powdery mildew in crops. Draculin an anticoagulant found in the saliva of vampire bats.[20] DuPhos A class of asymmetric ligands for asymmetric synthesis. The name DuPhos is derived from the chemical company that developed this type of ligand (DuP , DuPont) and the compound class of phospholanes (Phos) it belongs to. Earthcide or Fartox (C6Cl5NO2) Also called 'Quintozene, some of the many names for pentachloronitrobenzene, a fungicide.[21] Fenestrane a class of compounds with a 'window pane motif' (the name fenestrane derives from the Latin word fenestra, meaning window), comprising four fused carbocycles centred on a quaternary carbon resulting a twice over spiro compound. The illustration at right shows a generic fenestrane as well as the specific examples [4,4,4,4]fenestrane and [5,5,5,5]fenestrane. Fenestranes are of considerable interest in theoretical chemistry though comparatively few have actually been synthesised. Fucitol (C6H14O5), an alcohol derived from Fucus vesiculosis, a North Atlantic seaweed. Its optical isomers are also called D-fuc-ol and L-fuc-ol. fucK the name of the gene that encodes L-fuculokinase, an enzyme that catalyzes a chemical reaction between L-fuculose, ADP, and L-fuculose-1-phosphate. Furfural Furfural is an industrial chemical compound derived from a variety of agricultural byproducts, including corncobs, oat and wheat bran, and sawdust. The name furfural comes from the Latin word furfur, meaning bran, referring to its usual source. Fluoboric Acid BF4H, tetrafluoroborate or tetrafluoroboric acid. Fukalite (Ca4Si2O6(CO3)(OH, F))2, a rare form of calcium silicocarbonate mined in the Fuka region of Japan. Gossypol a toxin found in cottonseed used as a male contraceptive. Hirsutene [22][23] is also named after an animal: a goat (Hircus), occasionally the molecule is depicted upside down[24][25] Homocubane An molecule synthesized from cubane. Horseradish peroxidase An enzyme used extensively in molecular biology applications primarily for its ability to amplify a weak signal and increase detectability of a target molecule. Irene Hantzsch-Widman nomenclature for a monocyclic, heterocyclic compound with three ring atoms.[26] Josiphos A well-known catalyst, named after Josi Puleo, the technician who first prepared it.[27] Mandyphos and Taniaphos also exist. Ladderane An organic molecule that looks like a ladder because it contains two or more fused rings of cyclobutane. Megaphone a ketone derived from the root of Aniba megaphylla.[28] Mimimycin an anthracycline antibiotic agent named after the character Mimì in La Bohème.[7] Miraculin a glycoprotein found in Miracle Fruit that makes sour foods taste sweet after contact with taste buds.[29] Moronic acid 3-oxoolean-18-en-28-oic acid, a natural triterpene Mucic acid a product of nitric acid oxidation of galactose or galactose-containing compounds Muscovite a phyllosilicate mineral of aluminium and potassium. Musettamycin an anthracycline antibiotic agent named after the character Musetta in La Bohème.[7] Naftazone (C11H9N3O2), a vasoprotective drug. The NAFTA free-trade zone is the area covered by the North American Free Trade Agreement.[30] Nonanal (C9H18O), an aldehyde derived from nonane. Olympiadane A mechanically-interlocked compound based on the topology for the Olympic rings. Orotic acid (pyrimidinecarboxylic acid), has been referred to as vitamin B13. PEPPSI PEPPSI is short for Pyridine-Enhanced Precatalyst Preparation Stabilization and Initiation.[31] Performic acid a strongly oxidizing acid related to formic acid. Periodic acid or per-iodic acid is pronounced /ˌpɜr.aɪˈɒdɨk/ purr-eye-od-ik and not /ˌpɪərɪˈɒdɨk/ peer-ee-od-ik. It refers to one of the two interconvertible species HIO4 (metaperiodic acid) or H5IO6 (orthoperiodic acid - illustrated at right). The per- prefix in the name denotes that iodine is present in its highest possible (+VII) oxidation state. Periplanone B A pheromone of the female American cockroach. Named after the scientific name of this species, Periplaneta americana, not because of periplanarity. Picket Fence Porphyrin 5,10,15,20-tetrakis(alpha,alpha,alpha-2-pivalamidophenyl)porphyrin, used to model heme enzyme active sites. Pikachurin a retinal protein named after Pokémon character / species Pikachu Prismane an isomer of benzene with the carbon atoms arranged in the shape of a triangular prism. Psicose (C6H12O6), a rare low-calorie sugar that provides 0.3% as much energy as sucrose. Putrescine a foul-smelling diamine produced by the putrefaction of dead animal tissue. Quadratic acid a square-shaped organic compound, also called squaric acid. R-CMP (R-cytodine monophosphate) a component of RNA, but also the acronym for the Royal Canadian Mounted Police. Ranasmurfin a blue protein from the foam nests of a tropical frog, named after the Smurfs. Rednose a sugar derived from the degradation of rudolphomycin.[7] Rhamnetin a flavonol dye derived from buckthorn (rhamnus).[32] Rhamnose a sugar naturally occurring in buckthorn (rhamnus). Rudolphomycin an anthracycline antibiotic agent named after the character Rodolfo (Rudolph) in La Bohème.[7][33] Ru(Tris)BiPy-on-a-stick shorthand form of (trans-1,4-bis[(4-pyridyl)ethenyl]benzene)(2,2'-bipyridine)ruthenium(II).[34] SEX An abbreviation of sodium ethyl xanthate,[35] it has structural formula CH3CH2OCS2Na, IUPAC name sodium O-ethylcarbonodithioate, and it is a flotation agent used in the mining industry; Skatole a substance of disagreeable odor that occurs in feces, but also in lower concentrations in flowers, orange blossoms, jasmine. Sonic hedgehog a protein named after Sonic the Hedgehog Spermine, Spermidine, polyamine growth factors involved in cellular metabolism. Thebacon Dihydrocodeinone enol acetate, an opioid analgesic or antitussive. Titanic acid the hydrated form of titanium dioxide. Traumatic acid a substance occurring in plants, with a role in healing damaged tissue. Uranate the chemical term for an oxide anion of the element uranium. Uranocene U(C8H8)2, a uranium sandwich compound similar to ferrocene (Fe(C5H5)2) with two co-ordinating aromatic and anionic cyclooctatetraenide rings sandwiching the U atom (formally in its +IV oxidation state). Vomitoxin a mycotoxine occurring in grains. See also
Footnotes
- ^ Godly, E.W. (1998). Chemical Nomenclature. Kluwer Academic Publishers. pp. 20. ISBN 0751404756.
- ^ Thurlow, Kevin (1998). Chemical Nomenclature. Kluwer Academic Publishers. xii. ISBN 0751404756.
- ^ A more extensive list held at Bristol University
- ^ Dennis, Ryan (1997). "Old MacDonald Named a Compound: Branched Enynenynols" (PDF). Journal of Chemical Education 74 (7): 782. doi:10.1021/ed074p782. http://www.jce.divched.org/Journal/Issues/1997/Jul/PlusSub/V74N07/p782.pdf. Retrieved 2007-08-16.
- ^ Prelog, V., Seiwerth,R. (1941). Berichte 74: 1644 and 1769.
- ^ Not to be confused with the fictional material adamantium
- ^ a b c d e f g Nettleton DE Jr, Balitz DM, Doyle TW, Bradner WT, Johnson DL, O'Herron FA, Schreiber RH, Coon AB, Moseley JE, Myllymaki RW, J Nat Prod. 1980 Mar-Apr;43(2):242–258.
- ^ G. Märkl and H. Hauptmann (1983-06-14). "Untersuchungen zur Chemie der Arsole 1,1-dichlor-1-R-λ5-arsole-1-chlorarsole 2,2′,5,5′-tetraphenyldiarsolyl (Studies on the chemistry of arsoles)". J. Organomet. Chem. 248 (3): 269–285. doi:10.1016/S0022-328X(00)98709-6.
- ^ Mikael P. Johansson and Jonas Jusélius (2005). "Arsole Aromaticity Revisited". Lett. Org. Chem. (3): 469–474. http://www.helsinki.fi/~mpjohans/science/abstracts/008-abstract.html.
- ^ Zimmerman, Howard E.;; Robert M. Paufler. "Bicyclo [2.2.2]octa-2,5,7-triene (barrelene), a unique cyclic six electron pi system". Journal of the American Chemical Society 82 (6): 1514–1515. doi:10.1021/ja01491a071.
- ^ Schleyer, Paul von Rague; Eiji Osawa, Michael G. B. Drew (1968). "Nonacyclo[11.7.1.12,18.03,16.04,13.05,10.06,14.07,11.015,20docosane, a bastard tetramantane"] (PDF). J. Am. Chem. Soc 90 (18): 5034–5036. doi:10.1021/ja01020a053. http://pubs.acs.org/cgi-bin/searchRedirect.cgi/jacsat/1968/90/i18/pdf/ja01020a053.pdf. Retrieved 2007-08-15.[dead link]
- ^ Kroto, H.W.; Heath, J.R.; O'Brien, S.C.; Curl, R.F.; Smalley, R.E. Nature, 1985, 318(6042), 162.
- ^ Haymet, A.D.J. J. Am. Chem. Soc. 1986, 108, 319.
- ^ Doering, W. von E.; Roth, W. R. (1963). "A Rapidly Reversible Degenerate Cope Rearrangement : Bicyclo[5.1.0octa-2,5-diene"]. Tetrahedron 19 (5): 715–737. doi:10.1016/S0040-4020(01)99207-5. http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6THR-42H7VSF-15&_user=10&_coverDate=12%2F31%2F1963&_alid=1417428637&_rdoc=2&_fmt=high&_orig=search&_cdi=5289&_sort=r&_docanchor=&view=c&_ct=2&_acct=C000050221&_version=1&_urlVersion=0&_userid=10&md5=3c55b6721e1cb8c14ac0982a08ac1365.
- ^ Ault, Addison (2001). "The Bullvalene Story. The Conception of Bullvalene, a Molecule That Has No Permanent Structure". J. Chem. Educ. 78 (7): 924. doi:10.1021/ed078p924. http://www.jce.divched.org/Journal/Issues/2001/Jul/abs924.html.
- ^ NORDENSTROM BE (1951). "Effect of cadaverine and lysine on the urinary excretion of piperidine in rabbits". Acta pharmacologica et toxicologica 7 (3): 287–296. doi:10.1111/j.1600-0773.1951.tb02870.x. PMID 14856760.
- ^ D.O. Chester and J.A. Elix, Australian Journal of Chemistry 32 (1979) 2565-2569 doi:10.1071/CH9792565
- ^ Verbrugge, P. A. (1977). "Unusual organic compounds. XXIV. Compounds with the formula (CH)n. (d). Synthesis of cubane, (CH)8; homocubanes". Chemie en Techniek (Amsterdam) 32 (4): 120–123.
- ^ R A Klein, G P Hazlewood, P Kemp, and R M Dawson, Biochem J. 1979 December 1; 183(3): 691–700.
- ^ Apitz-Castro R, Béguin S, Tablante A, Bartoli F, Holt JC, Hemker HC (1995). "Purification and partial characterization of draculin, the anticoagulant factor present in the saliva of vampire bats (Desmodus rotundus)". Thromb. Haemost. 73 (1): 94–100. PMID 7740503.
- ^ "NIST Standard Reference Database 69, June 2005 Release: NIST Chemistry WebBook - Pentachloronitrobenzene". http://webbook.nist.gov/cgi/cbook.cgi?Name=fart*&Units=SI&cTG=on&cTC=on&cTP=on&cTR=on. Retrieved 2007-08-16.
- ^ Tandem radical approach to linear condensed cyclopentanoids. Total synthesis of (.+-.)-hirsutene Dennis P. Curran and Donna M. Rakiewicz J. Am. Chem. Soc.; 1985; 107(5) pp 1448 - 1449; doi:10.1021/ja00291a077
- ^ Isolation, structure and synthesis of hirsutene, a precursor hydrocarbon of coriolin biosynthesis Shigeo Nozoe, Jun Furukawa, Ushio Sankawa and Shoji Shibata Tetrahedron Letters Volume 17, Issue 3 , January 1976, Pages 195-198 doi:10.1016/0040-4039(76)80013-5
- ^ Hirsutene upside down
- ^ Hirsutene upside down
- ^ (PDF) Parent Hydride Names and Substantive Nomenclature. IUPAC. March 2004. pp. 16. http://www.iupac.org/reports/provisional/abstract04/RB-prs310804/Chap6-3.04.pdf.
- ^ Blaser, Hans-Ulrich; Brieden, Walter; Pugin, Benoit; Spindler, Felix; Studer, Martin; Togni, Antonio (2002). "Solvias Josiphos ligands: from discovery to technical applications". Topics in Catalysis 19 (1): 3–16. doi:10.1023/A:1013832630565.
- ^ SM Kupchan, KL Stevens, EA Rohlfing, BR Sickles, AT Sneden, RW Miller, RF Bryan, J. Org. Chem., 43(4) (1978) 586
- ^ Theerasilp S, Kurihara Y (August 1988). "Complete purification and characterization of the taste-modifying protein, miraculin, from miracle fruit". J. Biol. Chem. 263 (23): 11536–11539. PMID 3403544. http://www.jbc.org/cgi/reprint/263/23/11536.
- ^ Charles O, Coolsaet B (1972). "[Prevention of hemorrhage in prostatic surgery. Apropos of the study of the hemostatic activity in prostatectomy of a new molecule: beta-naphthoquinone monosemicarbazone (Naftazone)]" (in French). Annales d'urologie 6 (3): 209–212. PMID 4562066.
- ^ "PEPPSI Catalysts". http://www.sigmaaldrich.com/chemistry/chemical-synthesis/technology-spotlights/peppsi.html. Retrieved 2009-04-01.
- ^ Uri J, Csoban G, Viragh E., Acta Physiol Hung. 1951;2(2):223-8.
- ^ "Canadian Patents Database CA 1110562: Anthracycline antibiotic designated RUDOLPHOMYCIN". http://patents1.ic.gc.ca/details?patent_number=1110562. Retrieved 2007-08-16.
- ^ Toma SH, Uemi M, Nikolaou S, Tomazela DM, Eberlin MN, Toma HE., Inorg Chem. 2004 May 31; 43(11):3521–3527
- ^ See, for example, N Okibe and DB Johnson, Biotechnology Letters, 2004 24(23), 2011–2016 doi:10.1023/A:1021118915720
References
- Paul May, "Molecules with Silly or Unusual Names," Imperial College Press, July 2008, ISBN 978-1848162075.
- Alex Nickon and Ernest F. Silversmith, "Organic Chemistry, the Name Game: Modern Coined Terms and Their Origins", Pergamon 1987. ISBN 0-08-034481-X.
- W. V. Metanomski, "Unusual Names Assigned to chemical substances", Chem. Int., 1987, 9, 211-215.
- J. Andraos, "Glossary of Coined Names & Terms Used in Science", York University, 2004.
- E.C. Alyea, "Metal Complexes of Ditertiary Arsines. Chapter in Transition Metal Complexes of Phosphorus, Arsenic and Antimony Ligands", MacMillan, 1973. Chapter: "Some amusing names of arsine ligands: edas, vdias, dam, ffars etc"
- Giles, P.M. (1999). "Revised Section F: Natural products and related compounds". Pure Appl. Chem. 71 (4): 587–643. doi:10.1351/pac199971040587. http://www.chem.qmul.ac.uk/iupac/sectionF/. Retrieved 2007-08-16.
External links
Categories:- Chemistry lists
- Lists of names
- Lists of things considered unusual
Wikimedia Foundation. 2010.