- Cadaverine
-
Cadaverine 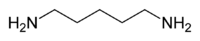
 pentane-1,5-diamine
pentane-1,5-diamineIdentifiers CAS number 462-94-2 
PubChem 273 ChemSpider 13866593 
UNII L90BEN6OLL 
DrugBank DB03854 KEGG C01672 
MeSH Cadaverine ChEBI CHEBI:18127 
ChEMBL CHEMBL119296 
Jmol-3D images Image 1 - NCCCCCN
Properties Molecular formula C5H14N2 Molar mass 102.178 Density 0.870 g/cm³ Melting point 9 °C
Boiling point 178-180 °C
 (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Cadaverine is a foul-smelling compound produced by protein hydrolysis during putrefaction of animal tissue. Cadaverine is a toxic[1] diamine with the formula NH2(CH2)5NH2, which is similar to putrescine. Cadaverine is also known by the names 1,5-pentanediamine and pentamethylenediamine.
Contents
History
Putrescine[2] and cadaverine[3] were first described in 1885 by the Berlin physician Ludwig Brieger (1849–1919).[4]
Production
Cadaverine is the decarboxylation product of the amino acid lysine.
However, this diamine is not purely associated with putrefaction. It is also produced in small quantities by living beings. It is partially responsible for the distinctive odors of urine and semen.
Clinical significance
Elevated levels of cadaverine have been found in the urine of some patients with defects in lysine metabolism.
Toxicity
Cadaverine is toxic in large doses. In rats it had an acute oral toxicity of more than 2000 mg/kg body weight.[5]
See also
Notes
- ^ Lewis 1998, Page 212
- ^ Ludwig Brieger, "Weitere Untersuchungen über Ptomaine" [Further investigations into ptomaines] (Berlin, Germany: August Hirschwald, 1885), page 43.
- ^ Ludwig Brieger, "Weitere Untersuchungen über Ptomaine" [Further investigations into ptomaines] (Berlin, Germany: August Hirschwald, 1885), page 39. From page 39: Ich nenne das neue Diamin C5H16N2: "Cadaverin", da ausser der empirischen Zussamsetzung, welche die neue Base als ein Hydrür des Neuridins für den flüchtigen Blick erscheinen lässt, keine Anhaltspunkte für die Berechtigung dieser Auffassung zu erheben waren. (I call the new di-amine, C5H16N2, "cadaverine," since besides its empirical composition, which allows the new base to appear superficially as a hydride of neuridine, no clues for the justification of this view arose.)
- ^ Brief biography of Ludwig Brieger (in German).
- ^ Acute and subacute toxicity of tyramine, spermidine, spermine, putrescine and cadaverine in rats
References
- Lewis, Robert Alan (1998). Lewis' Dictionary of Toxicology. CRC Press. ISBN 1566702232.
Categories:- Polyamines
- Foul-smelling chemicals
Wikimedia Foundation. 2010.
