- Moronic acid
-
Moronic acid 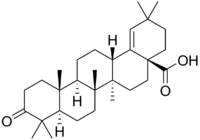 (4aS,6aR,6aS,6bR,8aS,12aS,14aS)-2,2,6a,6b,9,9,12a-heptamethyl-10-oxo-4,5,6,6a,7,8,8a,11,12,13,14,14a-dodecahydro-3H-picene-4a-carboxylic acidOther namesAmbronic acid; 3-Oxoolean-18-en-28-oic acid
(4aS,6aR,6aS,6bR,8aS,12aS,14aS)-2,2,6a,6b,9,9,12a-heptamethyl-10-oxo-4,5,6,6a,7,8,8a,11,12,13,14,14a-dodecahydro-3H-picene-4a-carboxylic acidOther namesAmbronic acid; 3-Oxoolean-18-en-28-oic acidIdentifiers CAS number 6713-27-5 
PubChem 489941 ChemSpider 429156 
ChEBI CHEBI:30815 
ChEMBL CHEMBL472646 
Jmol-3D images Image 1 - O=C2C([C@@H]1CC[C@@]4([C@@H]([C@@]1(C)CC2)CC[C@@H]5/C3=C/C(C)(C)CC[C@]3(C(=O)O)CC[C@@]45C)C)(C)C
- InChI=1S/C30H46O3/c1-25(2)14-16-30(24(32)33)17-15-28(6)19(20(30)18-25)8-9-22-27(5)12-11-23(31)26(3,4)21(27)10-13-29(22,28)7/h18-19,21-22H,8-17H2,1-7H3,(H,32,33)/t19-,21+,22-,27+,28-,29-,30+/m1/s1

Key: UMYJVVZWBKIXQQ-QALSDZMNSA-N
InChI=1/C30H46O3/c1-25(2)14-16-30(24(32)33)17-15-28(6)19(20(30)18-25)8-9-22-27(5)12-11-23(31)26(3,4)21(27)10-13-29(22,28)7/h18-19,21-22H,8-17H2,1-7H3,(H,32,33)/t19-,21+,22-,27+,28-,29-,30+/m1/s1
Key: UMYJVVZWBKIXQQ-QALSDZMNBW
Properties Molecular formula C30H46O3 Molar mass 454.68 g mol−1  acid (verify) (what is:
acid (verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Moronic acid (3-oxoolean-18-en-28-oic acid) is a natural triterpene.[1][2] Moronic acid can be extracted from Rhus javanica, a sumac plant traditionally believed to hold medicinal applications.[2] The molecule has also been extracted from Mistletoe (Phoradendron reichenbachianum).[3]
Bevirimat, a derivative of the related triterpenoid betulinic acid, is under development as an anti-HIV drug; however, moronic acid has shown better antiviral profiles in vitro than bevirimat.[4] A particular moronic acid derivative showed potent anti-HIV activity with EC50 values of 0.0085 μM against NL4-3, 0.021 μM against PI-R (a multiple protease inhibitor resistant strain), and 0.13 μM against FHR-2 (an HIV strain resistant to bevirimat). This derivative has become a new lead for clinical trials and is also active against herpes simplex virus 1.[4]
References
- ^ "Comparative Toxicogenomics Database: moronic acid". http://ctdbase.org/detail.go?type=chem&acc=C023607.
- ^ a b Kurokawa, Masahiko; Basnet, Purusotam; Ohsugi, Mizue; Hozumi, Toyoharu; Kadota, Shigetoshi; Namba, Tsuneo; Kawana, Takashi; Shiraki, Kimiyasu (1999). "Anti-Herpes Simplex Virus Activity of Moronic Acid Purified from Rhus javanica In Vitro and In Vivo". The Journal of Pharmacology and Experimental Therapeutics 289 (1): 72–8. PMID 10086989. http://jpet.aspetjournals.org/cgi/pmidlookup?view=long&pmid=10086989.
- ^ Rios, María Yolanda; Salinas, David; Villarreal, María Luisa (2001). "Cytotoxic Activity of Moronic Acid and Identification of the New Triterpene 3,4-seco-Olean-18-ene-3,28-dioic Acid from Phoradendron reichenbachianum". Planta Medica 67 (5): 443–6. doi:10.1055/s-2001-15823. PMID 11488459.
- ^ a b Yu, Donglei; Sakurai, Yojiro; Chen, Chin-Ho; Chang, Fang-Rong; Huang, Li; Kashiwada, Yoshiki; Lee, Kuo-Hsiung (2006). "Anti-AIDS Agents 69. Moronic Acid and Other Triterpene Derivatives as Novel Potent Anti-HIV Agents". Journal of Medicinal Chemistry 49 (18): 5462–9. doi:10.1021/jm0601912. PMC 2512972. PMID 16942019. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2512972.
External links
Categories:- Carboxylic acids
- Triterpenes
- Ketones
Wikimedia Foundation. 2010.
