- Buckminsterfullerene
-
Buckminsterfullerene 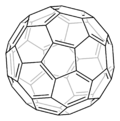
 (C60-Ih)[5,6]fullereneOther namesBuckyball; Fullerene-C60; [60]fullerene
(C60-Ih)[5,6]fullereneOther namesBuckyball; Fullerene-C60; [60]fullereneIdentifiers CAS number 99685-96-8 
PubChem 123591 ChemSpider 110185 
ChEBI CHEBI:33128 
Jmol-3D images Image 1 - c12c3c4c5c1c6c7c8c2c9c1c3c2c3c4c4c%10c5c5c6c6c7c7c%11c8c9c8c9c1c2c1c2c3c4c3c4c%10c5c5c6c6c7c7c%11c8c8c9c1c1c2c3c2c4c5c6c3c7c8c1c23
- InChI=1S/C60/c1-2-5-6-3(1)8-12-10-4(1)9-11-7(2)17-21-13(5)23-24-14(6)22-18(8)28-20(12)30-26-16(10)15(9)25-29-19(11)27(17)37-41-31(21)33(23)43-44-34(24)32(22)42-38(28)48-40(30)46-36(26)35(25)45-39(29)47(37)55-49(41)51(43)57-52(44)50(42)56(48)59-54(46)53(45)58(55)60(57)59

Key: XMWRBQBLMFGWIX-UHFFFAOYSA-N
InChI=1/C60/c1-2-5-6-3(1)8-12-10-4(1)9-11-7(2)17-21-13(5)23-24-14(6)22-18(8)28-20(12)30-26-16(10)15(9)25-29-19(11)27(17)37-41-31(21)33(23)43-44-34(24)32(22)42-38(28)48-40(30)46-36(26)35(25)45-39(29)47(37)55-49(41)51(43)57-52(44)50(42)56(48)59-54(46)53(45)58(55)60(57)59
Key: XMWRBQBLMFGWIX-UHFFFAOYAU
InChI=1S/C60/c1-2-5-6-3(1)8-12-10-4(1)9-11-7(2)17-21-13(5)23-24-14(6)22-18(8)28-20(12)30-26-16(10)15(9)25-29-19(11)27(17)37-41-31(21)33(23)43-44-34(24)32(22)42-38(28)48-40(30)46-36(26)35(25)45-39(29)47(37)55-49(41)51(43)57-52(44)50(42)56(48)59-54(46)53(45)58(55)60(57)59
Key: XMWRBQBLMFGWIX-UHFFFAOYSA-N
Properties Molecular formula C60 Molar mass 720.64 g mol−1  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references  Solution of buckminsterfullerene in benzene
Solution of buckminsterfullerene in benzene
Buckminsterfullerene is a spherical fullerene molecule with the formula C60. It was first intentionally prepared in 1985 by Harold Kroto, James Heath, Sean O'Brien, Robert Curl and Richard Smalley at Rice University.[1] Kroto, Curl, and Smalley were awarded the 1996 Nobel Prize in Chemistry for their roles in the discovery of buckminsterfullerene and the related class of molecules, the fullerenes. The name is a homage to Richard Buckminster Fuller, whose geodesic domes it resembles. Buckminsterfullerene was the first fullerene molecule discovered and it is also the most common in terms of natural occurrence, as it can be found in small quantities in soot.[2][3][4]
Buckminsterfullerene is the largest matter to have been shown to exhibit wave–particle duality.[5]
Structure
The structure of a buckminsterfullerene is a truncated icosahedron made of 20 hexagons and 12 pentagons, with a carbon atom at the vertices of each polygon and a bond along each polygon edge. The van der Waals diameter of a C60 molecule is about 1.01 nanometer (nm). The nucleus to nucleus diameter of a C60 molecule is about 0.71 nm. The C60 molecule has two bond lengths. The 6:6 ring bonds (between two hexagons) can be considered "double bonds" and are shorter than the 6:5 bonds (between a hexagon and a pentagon). Its average bond length is 1.4 Å (angstroms). Each carbon atom in the structure is bonded covalently with 3 others. Carbon atoms have 6 electrons, meaning their electronic structure is u2,4. To become stable, the carbon atom needs 8 electrons in its outer shell, and covalently bonding with 3 other atoms will only make 7 electrons in its outer shell. This means that the one unbonded electron on every carbon atom is free to float around all of the compound's atoms. This, in addition to its size, makes it potentially useful in nanotechnology.
References
- ^ Kroto, H.W.; et al. (1985). "C60: Buckminsterfullerene". Nature 318 (6042): 162–163. Bibcode 1985Natur.318..162K. doi:10.1038/318162a0.
- ^ Howard JB, McKinnon JT, Makarovsky Y, Lafleur AL, Johnson ME, in Nature 1991;352:139
- ^ Howard JB, Lafleur AL, Makarovsky Y, Mitra S, Pope CJ, Yadav TK, in Carbon 1992;30:1183
- ^ Grieco WJ, Lafleur AL, Swallow KC, Richter H, Taghizadeh K, Howard JB, in Proc. Combust Inst, 1998;27:1669
- ^ Nature: Wave–particle duality of C60 molecules, 14 October 1999. Abstract, subscription needed for full text
External links
 Media related to Fullerenes at Wikimedia Commons
Media related to Fullerenes at Wikimedia CommonsAllotropes of carbon sp3 forms sp2 forms sp forms Linear acetylenic carbonmixed sp3/sp2 forms other forms related Categories:- Fullerenes
Wikimedia Foundation. 2010.
