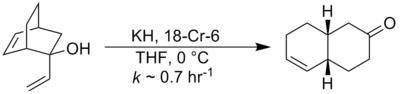- Cope rearrangement
-
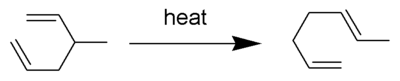
The Cope rearrangement is an extensively studied organic reaction involving the [3,3]-sigmatropic rearrangement of 1,5-dienes.[1][2][3][4] It was developed by Arthur C. Cope. For example 3-methyl-1,5-hexadiene heated to 300°C yields 1,5-heptadiene.
The Cope rearrangement causes the fluxional states of the molecules in the bullvalene family.
Contents
Mechanism
Although the Cope rearrangement is concerted and pericyclic, it can also be considered to go via a transition state that is energetically and structurally equivalent to a diradical. This is an alternative explanation which remains faithful to the uncharged nature of the Cope transition state, while preserving the principles of orbital symmetry. This also explains the high energy requirement to perform a Cope rearrangement. Although illustrated in the chair conformation, the Cope can also occur with cyclohexadienes in the "boat" conformation.

The above description of the transition state is not quite correct. It is currently generally accepted that the Cope rearrangement follows an allowed concerted route through a homoaromatic transition state and not a diradical. That is unless the potential energy surface is perturbed to favor the diradical.[5]
Examples
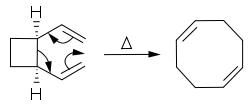
The rearrangement is widely used in organic synthesis. It is symmetry-allowed when it is suprafacial on all components. The transition state of the molecule passes through a boat or chair like transition state. An example of the Cope rearrangement is the expansion of a cyclobutane ring to a 1,5-cyclooctadiene ring:
In this case, the reaction must pass through the boat transition state to produce the two cis double bonds. A trans double bond in the ring would be too strained. The reaction occurs under thermal conditions. The driving force of the reaction is the loss of strain from the cyclobutane ring.
Oxy-Cope rearrangement
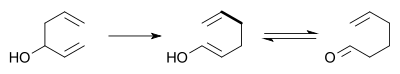
In the Oxy-Cope rearrangement a hydroxyl group is added at C3 forming a enal or enone after Keto-enol tautomerism of the intermediate enol [6][7]:
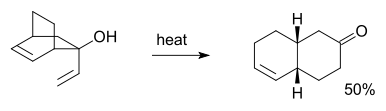
for instance in this reaction:
In 1975, Evans and Golob showed that deprotonation of oxy-Cope substrates to form the corresponding alkali metal alkoxides resulted in rate accelerations of 1010 to 1017 for the oxy-Cope rearrangement. Typically potassium hydride and 18-crown-6 are employed in order to generate a fully dissociated potassium alkoxide[8] :
It is noteworthy that the anion-accelerated oxy-Cope reaction can proceed with high efficiency even in systems that do not permit good orbital overlap, as seen in this example from Schreiber's synthesis periplanone B[9] :
The authors remark that the corresponding neutral oxy-Cope and siloxy-Cope rearrangements failed, giving only elimination products at 200 °C.
Variations
Another variation of the Cope rearrangement is the heteroatom Cope reactions such as the Aza-Cope rearrangement. Another widely studied [3, 3] sigmatropic rearrangement is the Claisen rearrangement. Also see the divinylcyclopropane-cycloheptadiene rearrangement.
References
- ^ Arthur C. Cope; et al.; J. Am. Chem. Soc. 1940, 62, 441.
- ^ Rhoads, S. J.; Raulins, N. R.; Org. React. 1975, 22, 1-252. (Review)
- ^ Hill, R. K.; Comp. Org. Syn. 1991, 5, 785-826.
- ^ Wilson, S. R.; Org. React. 1993, 43, 93-250. (Review)
- ^ Williams, R. V., Chem. Rev. 2001, 101 (5), 1185-1204.
- ^ A Synthesis of Ketones by the Thermal Isomerization of 3-Hydroxy-1,5-hexadienes. The Oxy-Cope Rearrangement Jerome A. Berson, Maitland Jones, , Jr. J. Am. Chem. Soc. 1964; 86(22); 5019-5020. doi:10.1021/ja01076a067
- ^ Stepwise Mechanisms in the Oxy-Cope Rearrangement Jerome A. Berson and Maitland Jones pp 5017 - 5018; J. Am. Chem. Soc. 1964; doi:10.1021/ja01076a066
- ^ Evans, D.A.; Golob, A.M. J. Am. Chem. Soc. 1975, 97, 4765-4766. doi:10.1021/ja00849a054
- ^ Schreiber, S.L.; Santini, S. J. Am. Chem. Soc. 1984, 106, 4038-4039. doi:10.1021/ja00326a028
Categories:- Rearrangement reactions
- Name reactions
Wikimedia Foundation. 2010.