- Cope reaction
-
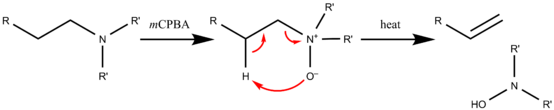
The Cope reaction or Cope elimination, developed by Arthur C. Cope, is an elimination reaction of an amine oxide to form an alkene and a hydroxylamine. The reaction mechanism involves an intramolcular 5-membered cyclic transition state,[1] leading to a syn elimination product. This organic reaction gives the same result as the Hofmann elimination,[2] but the base is a part of the leaving group. The amine oxide is prepared by oxidation of the corresponding amine with an oxidant such as mCPBA. The actual elimination just requires heat.
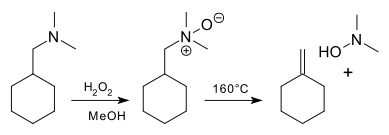
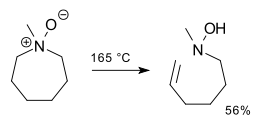
An application is a synthesis of methylenecyclohexane [1]:Piperidines are resistant to an intramolecular Cope reaction [2] [3] [4] but with pyrrolidine and with rings of size 7 and larger, the reaction product is an unsaturated hydroxyl amine. This result demonstrates the geometric constraints of a 5-membered cyclic transition state.
References
- ^ Organic Syntheses, Coll. Vol. 4, p.612 (1963); Vol. 39, p.40 (1959). Link
- ^ March, Jerry (1985), Advanced Organic Chemistry: Reactions, Mechanisms, and Structure (3rd ed.), New York: Wiley, ISBN 0-471-85472-7
- ^ Amine Oxides. VIII. Medium-sized Cyclic Olefins from Amine Oxides and Quaternary Ammonium Hydroxides Arthur C. Cope, Engelbert Ciganek, Charles F. Howell, Edward E. Schweizer J. Am. Chem. Soc., 1960, 82 (17), pp 4663–4669 doi:10.1021/ja01502a053
- ^ Amine Oxides. VII. The Thermal Decomposition of the N-Oxides of N-Methylazacycloalkanes Arthur C. Cope, Norman A. LeBel; J. Am. Chem. Soc.; 1960; 82(17); 4656-4662. doi:10.1021/ja01502a052
Categories:- Elimination reactions
- Name reactions
Wikimedia Foundation. 2010.