- Amine oxide
-
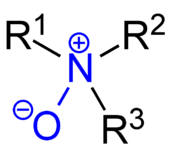
An amine oxide, also known as amine-N-oxide and N-oxide, is a chemical compound that contains the functional group R3N+-O−, an N-O bond with three additional hydrogen and/or hydrocarbon side chains attached to N. Sometimes it is written as R3N→O or, wrongly, as R3N=O.
In the strict sense the term amine oxide applies only to oxides of tertiary amines. Sometimes it is also used for the analogous derivatives of primary and secondary amines.
Examples of amine oxides include pyridine N-oxide, a water-soluble crystalline solid with melting point 62-67°C, and N-methylmorpholine N-oxide, which is an oxidant.
Contents
Properties
Amine oxides are used as protecting group for amines and as chemical intermediates. Long-chain alkyl amine oxides are used as nonionic surfactants and foam stabilizers.
Amine oxides are highly polar molecules have a polarity close to that of quaternary ammonium salts. Small amine oxides are very hydrophilic and have an excellent water solubility and a very poor solubility in most organic solvents.
Amine oxides are weak bases with a pKa of around 4.5 that form R3N+-OH, cationic hydroxylamines, upon protonation at a pH below their pKa.
Synthesis
Amine oxides are prepared by oxidation of tertiary amines or pyridine analogs with hydrogen peroxide (H2O2), Caro's acid or peracids like mCPBA in N-oxidation [1].
Reactions
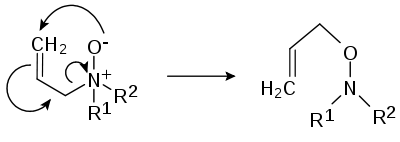
- Pyrolytic elimination. Amine oxides, when heated to 150 to 200 °C eliminate a hydroxylamine, resulting in an alkene. This pyrolytic syn-elimination reaction is known under the name Cope reaction. The mechanism is similar to that of the Hofmann elimination.
- Reduction to amines. Amine oxides are readily converted to the parent amine by common reduction reagents including lithium aluminium hydride, sodium borohydride, catalytic reduction, zinc / acetic acid, and iron / acetic acid. Pyridine N-oxides can be deoxygenated by phosphorus oxychloride
- Sacrificial catalysis. Oxidants can be regenerated by reduction of N-oxides, as in the case of regeneration of osmium tetroxide by N-methylmorpholine oxide.
- O-alkylation. Pyridine N-oxides react with alkyl halides to the O-alkylated product
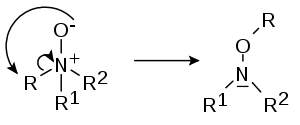
- In the Meisenheimer rearrangement (after Jakob Meisenheimer) certain N-oxides R1R2R3N+O- rearrange to hydroxylamines R2R3N-O-R1 [2][3]
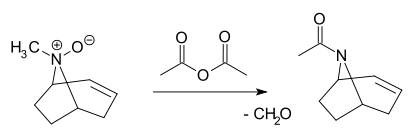
- In the Polonovski reaction a tertiary N-oxide is cleaved by acetic acid anhydride to the corresponding acetamide and aldehyde[4][5][6]:
Metabolites
Amine oxides are common metabolites of medication and psychoactive drugs. Examples include nicotine, Zolmitriptan, and morphine.
Amine oxides of anti-cancer drugs have been developed as prodrugs that are metabolized in the oxygen deficient cancer tissue to the active drug.
See also
- Functional group
- Amine, NR3
- Hydroxylamine, NR2OH
- Phosphine oxide, PR3=O
- Sulfoxide, R2S=O
- Azoxy, RN=N+(O–)R RN=N+RO−
- TEMPO (2,2,6,6-Tetramethylpiperidine-1-oxyl), a stable amine oxide radical
References
- ^ Recent trends in the chemistry of pyridine N-oxides Shaker Youssif Arkivoc 2001 Link
- ^ J. Meisenheimer, Ber. 52. 1667 (1919)
- ^ March's Advanced Organic Chemistry: Reactions, Mechanisms, and Structure Michael B. Smith, Jerry March Wiley-Interscience, 5th edition, 2001, ISBN 0-471-58589-0
- ^ Grierson, D. Org. React. 1990, 39, 85. (doi: 10.1002/0471264180.or039.02)
- ^ M. Polonovski, M. Polonovski, Bull. Soc. Chim. France 41, 1190 (1927).
- ^ Strategic Applications of Named Reactions in Organic Synthesis (Paperback) by Laszlo Kurti, Barbara Czako ISBN 0-12-429785-4
External links
Categories:- Amine oxides
- Functional groups
Wikimedia Foundation. 2010.