- N-Methylmorpholine N-oxide
-
N-Methylmorpholine N-oxide 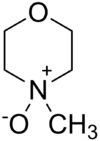
Identifiers CAS number 7529-22-8 
PubChem 82029 ChemSpider 74032 
ChEBI CHEBI:52093 
Jmol-3D images Image 1 - C[N+]1(CCOCC1)[O-]
Properties Molecular formula C5H11NO2 Molar mass 117.15 g/mol Melting point 180 - 184 °C
 (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references N-Methylmorpholine-N-oxide, NMO or NMMO is an organic compound. This heterocyclic amine oxide and morpholine derivative is used in organic chemistry as a co-oxidant and sacrificial catalyst in oxidation reactions for instance in osmium tetroxide oxidations and the Sharpless asymmetric dihydroxylation or oxidations with TPAP.[1] NMO is commercially supplied both as a monohydrate C5H11NO2.H2O and as the anhydrous compound. The monohydrate is used as a solvent for cellulose in the Lyocell process to produce cellulose fibers.
Contents
Uses
Solvent of cellulose
NMMO monohydrate is used as a solvent in the Lyocell process to produce Tencel fiber.[2] It dissolves cellulose to form a solution called dope, and the cellulose is reprecipitated in a water bath to produce a fiber. The process is similar but not analogous to the viscose process. In the viscose process, cellulose is made soluble by conversion to its xanthate derivatives. With NMMO, cellulose is not derivatized but dissolves to give a homogeneous polymer solution. The resulting fiber is similar to viscose; this was observed, for example, for Valonia cellulose microfibrils. Dilution with water causes the cellulose to reprecipitate, i.e. the solvation of cellulose with NMMO is a water sensitive process.[3]
Cellulose remains insoluble to most solvents since it has a strong and highly structured intermolecular hydrogen bonding network, which resists common solvents. NMMO is able to break the hydrogen bonding network that keeps cellulose insoluble to water and other solvents. Similar solubility has been obtained in a few solvents, particularly a mix of lithium chloride in dimethyl acetamide and some hydrophilic ionic liquids.
Dissolution of scleroproteins
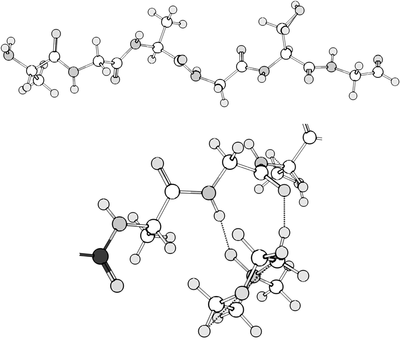 Hydrogen bonding between hexapeptide and NMO[4]
Hydrogen bonding between hexapeptide and NMO[4]
Another use of NMO is in the dissolution of scleroprotein (found in animal tissue). This dissolution occurs in the crystal areas which are more homogeneous and contain glycine and alanine residues with a small number of other residues. How NMO dissolves these proteins is scarcely studied. Other studies, however, have been done in similar amide systems (i.e. hexapeptide). The hydrogen bonds of the amides can be broken by NMO.[4]
Oxidant
 Oxidation of an alkene with osmium tetroxide (0.06 eq.) and NMO (1.2 eq.)in acetone/water 5:1 RT 12 hrs.[5]
Oxidation of an alkene with osmium tetroxide (0.06 eq.) and NMO (1.2 eq.)in acetone/water 5:1 RT 12 hrs.[5]NMO, as a N-oxide, is an oxidant. It is used as a stoichiometric oxidant to regenerate the main catalyst after the main catalyst has been reduced by the substrate. For example, the toxic and volatile osmium tetroxide needs to be added in stoichiometric amounts to effect dihydroxylation, but if continuously regenerated with NMO, the amount can be reduced to catalytic quantities.
References
- ^ Mark R. Sivik and Scott D. Edmondson "N-Methylmorpholine N-Oxide" E-EROS ENCYCLOPEDIA OF REAGENTS FOR ORGANIC SYNTHESIS, 2008 doi:10.1002/047084289X.rm216.pub2
- ^ Hans Krässig, Josef Schurz, Robert G. Steadman, Karl Schliefer, Wilhelm Albrecht, Marc Mohring, Harald Schlosser "Cellulose" in Ullmann's Encylopedia of Industrial Chemistry, 2002, Wiley-VCH, Weinheim. doi:10.1002/14356007.a05_375.pub2
- ^ Noé, Pierre, and Henri Chanzy "Swelling of Valonia cellulose microfibrils in amine oxide systems." Canadian Journal of Chemistry Volume 86 issue 6 pages 520-524(2008). retrieved EBSCO, Advanced Placement Source. 11 Nov. 2009.
- ^ a b E. S. Sashina, N. P. Novoselov, S. V.Toroshekova, V. E. Petrenko, “Quantum-chemical study of the mechanism of dissolution of scleroproteins in N-methylmorpholine N-oxide.” Russian Journal of General Chemistry volume78 issue 1 pages 139-145 (2008). retrieved EBSCO, Advanced Placement Source. 11 Nov. 2009.
- ^ Preparation of 3H-Pyrrolo[2,3-c]isoquinolines and 3H-Pyrrolo[2,3-c][2,6]- and 3H-Pyrrolo[2,3-c][1,7]-naphthyridines U. Narasimha Rao, Xuemei Han and Edward R. Biehl Arkivoc 2002 (x) 61-66 Online Article
Categories:- Morpholines
- Amine oxides
- Reagents for organic chemistry
Wikimedia Foundation. 2010.
