- DuPhos
-
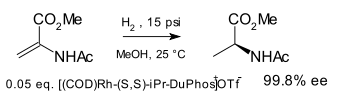
DuPhos is a class of asymmetric ligands for asymmetric synthesis. The name DuPhos is derived from the chemical company that developed this type of ligand (DuP, DuPont) and the compound class of phospholanes (Phos) it belongs to. This diphosphine ligand type was introduced in 1991 by M.J. Burk [1][2] and first demonstrated in asymmetric hydrogenation of certain enamide esters to amino acid precursors:
Other phosphine asymmetric ligands were known at the time (DIPAMP, BINOL, CHIRAPHOS) but the new ligand was found to be more effective.
Contents
Description
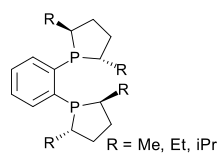
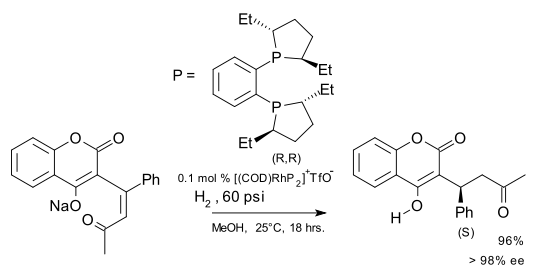
The ligand consists of two 2,5-alkyl-substituted phospholane rings (the phosphorus analog of THF) connected via a 1,2-phenyl bridge. The alkyl group can be methyl, ethyl, propyl or isopropyl. In the closely related bis(dimethylphospholano)ethane or BPE ligand [3][4] the phenyl bridge is replaced by an 1,2-ethyl bridge. Both compounds can be obtained from the corresponding chiral diol through conversion to the cyclic sulfate and reaction with lithiated phenylbisphosphine. In DuPhos the phosphorus atoms are electron-rich making the resulting metal complexes reactive. The phosphorus atoms also introduce a kind of pseudo-chirality making enantioselection independent of the overall chemical conformation [5]
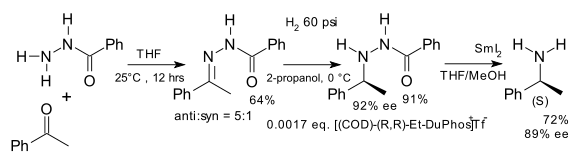
Another early application is the synthesis of unnatural chiral amino acids in a formal reductive amination [6] for example starting from benzophenone and the hydrazone of benzoyl chloride [7]:
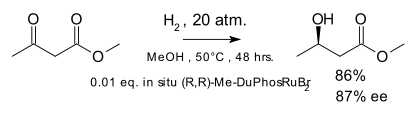
In the original scope the metal catalyst was rhodium but catalysis by ruthenium was introduced in 1995 [8] with the hydrogenation of the ketone group in β-keto esters:
Applications
An application of an asymmetric synthesis with a DuPhos ligand is the hydrogenation of dehydrowarfarin to warfarin [9]:
Duphos is also applied in the synthesis of tryptophan derivatives.[10]
In polymerization catalysis
DuPhos ligands are used in metal catalyzed alpha-olefin / carbon monoxide copolymerization to form chiral isotactic polyketones. The first publication in this field dates back to 1994 with catalyst system [Pd(Me-DuPhos(MeCN)2)](BF4)2 [11]
BozPhos ligand
Mono oxidation of (R,R)-Me-Duphos using borane dimethylsulfide as protective group and hydrogen peroxide as oxidizing agent gives bozPhos [12][13] This ligand is useful in copper-catalyzed asymmetric addition of diorganozinc reagents to N-diphenylphosphinoylimines.
References
- ^ C2-symmetric bis(phospholanes) and their use in highly enantioselective hydrogenation reactions Mark J. Burk J. Am. Chem. Soc., 1991, 113 (22), pp 8518–8519 doi:10.1021/ja00022a047
- ^ Preparation and use of C2-symmetric bis(phospholanes): production of .alpha.-amino acid derivatives via highly enantioselective hydrogenation reactions Mark J. Burk, John E. Feaster, William A. Nugent, Richard L. Harlow J. Am. Chem. Soc., 1993, 115 (22), pp 10125–10138 doi:10.1021/ja00075a031
- ^ New electron-rich chiral phosphines for asymmetric catalysis Mark J. Burk, John E. Feaster, Richard L. Harlow Organometallics, 1990, 9 (10), pp 2653–2655 doi:10.1021/om00160a010
- ^ New chiral phospholanes; Synthesis, characterization, and use in asymmetric hydrogenation reactions Tetrahedron: Asymmetry, Volume 2, Issue 7, 1991, Pages 569-592 Mark J. Burk, John E. Feaster, Richard L. Harlow doi:10.1016/S0957-4166(00)86109-1
- ^ Recent Developments in Catalytic Asymmetric Hydrogenation Employing P-Chirogenic Diphosphine Ligands Karen V. L. Crépy, Tsuneo Imamoto Advanced Synthesis & Catalysis Volume 345 Issue 1-2, Pages 79 - 101 2003 doi:10.1002/adsc.200390031
- ^ Enantioselective hydrogenation of the C:N group: a catalytic asymmetric reductive amination procedure Mark J. Burk, John E. Feaster J. Am. Chem. Soc., 1992, 114 (15), pp 6266–6267 doi:10.1021/ja00041a067
- ^ Catalytic asymmetric reductive amination of ketones via highly enantioselective hydrogenation of the C=N double bond Mark J. Burk , Jose P. Martinez, John E. Feaster and Nick Cosford Tetrahedron Volume 50, Issue 15, 11 April 1994, Pages 4399-4428 doi:10.1016/S0040-4020(01)89375-3
- ^ Practical asymmetric hydrogenation of β-keto esters at atmospheric pressure using chiral Ru (II) catalysts J. P. Genêt, V. Ratovelomanana-Vidal, M. C. Caño de Andrade, X. Pfister, P. Guerreiro and J. Y. Lenoir Tetrahedron Letters Volume 36, Issue 27, 3 July 1995, Pages 4801-4804 doi:10.1016/0040-4039(95)00873-B
- ^ The first practical asymmetric synthesis of R and S-Warfarin Andrea Robinson and Hui-Yin Li John Feaster Tetrahedron Letters Volume 37, Issue 46, 11 November 1996, Pages 8321-8324 doi:10.1016/0040-4039(96)01796-0
- ^ A highly enantioselective asymmetric hydrogenation route to β-(2R,3S)-methyltryptophan R. Scott Hoerrner, David Askin, R.P. Volante and Paul J. Reider Tetrahedron Letters Volume 39, Issue 21, 21 May 1998, Pages 3455-3458 doi:10.1016/S0040-4039(98)00604-2
- ^ Palladium(II)-Catalyzed Isospecific Alternating Copolymerization of Aliphatic .alpha.-Olefins with Carbon Monoxide and Isospecific Alternating Isomerization Cooligomerization of a 1,2-Disubstituted Olefin with Carbon Monoxide. Synthesis of Novel, Optically Active, Isotactic 1,4- and 1,5-Polyketones Zhaozhong Jiang, Ayusman Sen J. Am. Chem. Soc., 1995, 117 (16), pp 4455–4467 doi:10.1021/ja00121a003
- ^ Alexandre Côté, Jean-Nicolas Desrosiers, Alessandro A. Boezio, and André B. Charette (2006), "Preparation of enantiomerically pure (R,R)-BozPhos", Org. Synth. 83: 1, http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=v83p0001
- ^ Jean-Nicolas Desrosiers, Alexandre Côté, Alessandro A. Boezio, and André B. Charette (2006), "Preparation of enantiomerically enriched (1S)-1-Phenylpropan-1-amine hydrochloride by a catalytic addition of diorganozinc reagents to imines", Org. Synth. 83: 5, http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=v83p0005
Categories: Named phosphines | Bisphosphanes
Wikimedia Foundation. 2010.