- Cerebral aneurysm
-
Cerebral aneurysm Classification and external resources 
Diagram of cerebral aneurysm.ICD-10 I67.1 ICD-9 437.3 MeSH D002532 A cerebral or brain aneurysm is a cerebrovascular disorder in which weakness in the wall of a cerebral artery or vein causes a localized dilation or ballooning of the blood vessel.
Contents
Signs and symptoms
A small, unchanging aneurysm will produce little, if any, symptoms. Before a larger aneurysm ruptures, the individual may experience such symptoms as a sudden and unusually severe headache, nausea, vision impairment, vomiting, and loss of consciousness, or the individual may be asymptomatic, experiencing no symptoms at all. [1][2]
If an aneurysm ruptures, it leaks blood into the space around the brain. This is called a “subarachnoid hemorrhage.” Depending on the amount of blood, it can produce:[3]
- a sudden severe headache that can last from several hours to days
- nausea and vomiting
- drowsiness and/or coma
The ruptured aneurism (hemorrhage) may also damage the brain directly, usually from bleeding into the brain itself. This is called a “hemorrhagic stroke.” This can lead to:[3]
- weakness or paralysis of an arm or leg
- trouble speaking or understanding language
- vision problems
- seizures
Causes
Aneurysms may result from congenital defects, preexisting conditions such as high blood pressure and atherosclerosis (the buildup of fatty deposits in the arteries), or head trauma. Cerebral aneurysms occur more commonly in adults than in children but they may occur at any age. They are more common in women than in men, by a ratio of 3 to 2.[1]
The pursuit to identify Genetics of Intracranial Aneurysms has identified a number of locations, most recently 1p34-36, 2p14-15, 7q11, 11q25, and 19q13.1-13.3.
Locations
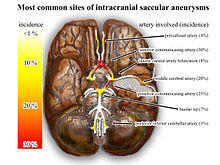
A common location of cerebral aneurysms is on the arteries at the base of the brain, known as the Circle of Willis. Approximately 85% of cerebral aneurysms develop in the anterior part of the Circle of Willis, and involve the internal carotid arteries and their major branches that supply the anterior and middle sections of the brain. The most common sites include the anterior cerebral artery and anterior communicating artery (30–35%), the bifurcation, division of two branches, of the internal carotid and posterior communicating artery (30–35%), the bifurcation of the middle cerebral artery (20%), the bifurcation of the basilar artery, and the remaining posterior circulation arteries (5%).
Onset and risks
Onset is usually sudden and without warning. Rupture of a cerebral aneurysm is dangerous and usually results in bleeding into the meninges or the brain itself, leading to a subarachnoid hemorrhage (SAH) or intracranial hematoma (ICH), either of which constitutes a stroke. Rebleeding, hydrocephalus (the excessive accumulation of cerebrospinal fluid), vasospasm (spasm, or narrowing, of the blood vessels), or multiple aneurysms may also occur. The risk of rupture from an unruptured cerebral aneurysm varies according to the size of an aneurysm, with the risk rising as the aneurysm size increases. The overall rate of aneurysm rupture is estimated at 1.3% per year, resulting in approximately 27,000 new cases of SAH in the United States per year. [1] Screening for aneurysms with annual imaging is possible, but not viewed as cost effective. [2] The risk of short term re-rupture decreases dramatically after an aneurysm has bled in about 3 days, though after approximately 6 weeks the risk returns to baseline.[citation needed]
Classification
Cerebral aneurysms are classified both by size and shape. Small aneurysms have a diameter of less than 15 mm. Larger aneurysms include those classified as large (15 to 25 mm), giant (25 to 50 mm), and super giant (over 50 mm). Saccular aneurysms are those with a saccular outpouching and are the least common form of cerebral aneurysm. Berry aneurysms are saccular aneurysms with necks or stems resembling a berry. Fusiform aneurysms are aneurysms without stems.
In outlining symptoms of ruptured cerebral aneurysm, it is useful to make use of the Hunt and Hess scale[4] of subarachnoid hemorrhage severity:
- Grade 1: Asymptomatic; or minimal headache and slight nuchal rigidity.
- Grade 2: Moderate to severe headache; nuchal rigidity; no neurologic deficit except cranial nerve palsy.
- Grade 3: Drowsy; minimal neurologic deficit.
- Grade 4: Stuporous; moderate to severe hemiparesis; possibly early decerebrate rigidity and vegetative disturbances.
- Grade 5: Deep coma; decerebrate rigidity; moribund.
- Grade 6: Death. By definition, someone who presents brain dead following SAH is grade 6.
The Fisher Grade[5] classifies the appearance of subarachnoid hemorrhage on CT scan:
- Grade 1: No hemorrhage evident.
- Grade 2: Subarachnoid hemorrhage less than 1 mm thick.
- Grade 3: Subarachnoid hemorrhage more than 1 mm thick.
- Grade 4: Subarachnoid hemorrhage of any thickness with intra-ventricular hemorrhage (IVH) or parenchymal extension.
The Fisher Grade is most useful in communicating the description of SAH and stratifies patients' risk for vasospasm. It is not intended to be used as a prognostic scale, unlike the Hunt-Hess scale.
Vasospasm
One complication of aneurysmal subarachnoid hemorrhage is the development of vasospasm. Approximately 1 to 2 weeks following the initial hemorrhage, patients may experience 'spasm' of the cerebral arteries, which can result in stroke. The etiology of vasospasm is thought to be secondary to an inflammatory process that occurs as the blood in the subarachnoid space is resorbed. It appears that macrophages and neutrophils that enter the subarachnoid space to phagocytose senescent erythrocytes and clear extracorpuscular hemoglobin, remain trapped in the subarachnoid space, die and degranulate 3–4 days after their arrival, and release massive quantities of endothelins and free radicals that in turn induce vasospasm.[6] Vascular narrowing, however, is only one component of the transient inflammatory injury, which is extensive.
Vasospasm is monitored in a variety of ways. Non-invasive methods include transcranial Doppler, which is a method of measuring the velocity of blood in the cerebral arteries using ultrasound. As the vessels narrow due to vasospasm, the velocity of blood increases. The amount of blood reaching the brain can also be measured by CT or MRI or nuclear perfusion scanning.
The definitive, but invasive method of detecting vasospasm is cerebral angiography. It is generally agreed that in order to prevent or reduce the risk of permanent neurological deficits, or even death, vasospasm should be treated aggressively. This is usually performed by early delivery of drug and fluid therapy, or 'Triple H' (hypertensive-hypervolemic-hemodilution therapy) (which elevates blood pressure, increases blood volume, and thins the blood) to drive blood flow through and around blocked arteries. For patients who are refractive (resistant) to Triple H therapy, narrowed arteries in the brain can be treated with medication delivered into the arteries that are in spasm and with balloon angioplasty to widen the arteries and increase blood flow to the brain. Although the effectiveness of these treatments is well established, angioplasty and other treatments delivered by interventional radiologists have been in evolution over the past several years. It is generally recommended that aneurysms be evaluated at specialty centers which provide both neurosurgical and interventional radiology treatment and which also permit angioplasty, if needed, without transfer.
Treatment
Emergency treatment for individuals with a ruptured cerebral aneurysm generally includes restoring deteriorating respiration and reducing intracranial pressure. Currently there are three treatment options for brain aneurysms: medical hypotensive therapy; surgical clipping or endovascular coiling. If possible, either surgical clipping or endovascular coiling is usually performed within the first 24 hours after bleeding to occlude the ruptured aneurysm and reduce the risk of rebleeding.
Hypotensive therapy
Medical—hypotensive therapy for ruptured intracranial aneurysms was introduced by Paul Slosberg MD ( currently in private practice) at the Mount Sinai Hospital in 1956 and was shown superior to surgery and other treatments in the largest randomized controlled study (multinational—15 institutions) ever conducted. This was reported in the major neurologic journal Stroke years ago but was underpublicized. More recently, with modifications for unruptured brain aneurysms and review of 50 years' results it has again been found superior to surgical and now also to endovascular treatment. The method has the extreme cost-benefit advantage of completely eliminating the need for hospitalization itself, thereby eliminating surgical costs, endovascular costs, operating room costs and recovery room costs. In addition, it enables patients to completely avoid life-threatening nosocomial i.e. hospital-based, infections especially the frequently fatal MRSA infections along with other fatal hospital-based infections now being reported. This entirely medical treatment is performed by the neurologist both early and in long-term follow-up, in a private office or outpatient hospital facility. Aneurysms have been treated successfully regardless of size(e.g. giant aneurysms are included), location, complicating medical illnesses etc. These long term clinical results are buttressed by long-term MRA and CTA radiographic results showing that instead of the expected increase in size, the aneurysms either remain the same size, decrease in size or are no longer even visualized. This entirely medical method has now been endorsed by least two aneurysm surgical groups in England, as reported in both the Journal of Neurosurgery and Lancet Neurology.
It is not considered standard management of acute intracranial aneurysm rupture in the UK.
Surgical clipping
Main article: Surgical clippingSurgical clipping was introduced by Walter Dandy of the Johns Hopkins Hospital in 1937. It consists of performing a craniotomy, exposing the aneurysm, and closing the base of the aneurysm with a clip chosen specifically for the site. The surgical technique has been modified and improved over the years. Surgical clipping has a lower rate of aneurysm recurrence after treatment.
In January 2009, a team of doctors at UNC Hospital in Chapel Hill, North Carolina pioneered a new approach for aneurysm treatment — clipping aneurysms through an endoscopic endonasal approach. The team was led by UNC neurosurgeon, Dr. Anand Germanwala. This procedure may be groundbreaking for patients with aneurysms near the skull base, as an approach through the nose is less invasive than traditional approaches. Two videos related to this procedure can be seen on the UNC Neurosurgery website: http://www.med.unc.edu/neurosurgery/news/germanwala-presents-first-aneurysm-patient-treated-through-nose and http://www.med.unc.edu/neurosurgery/news/video-it-takes-two-or-more [7]
Endovascular coiling
Endovascular coiling was introduced by Guido Guglielmi at UCLA in 1991. It consists of passing a catheter into the femoral artery in the groin, through the aorta, into the brain arteries, and finally into the aneurysm itself. Once the catheter is in the aneurysm, platinum coils are pushed into the aneurysm and released. These coils initiate a clotting or thrombotic reaction within the aneurysm that, if successful, will eliminate the aneurysm. These procedures require a small incision, through which a catheter is inserted. In the case of broad-based aneurysms, a stent may be passed first into the parent artery to serve as a scaffold for the coils ("stent-assisted coiling"), although the long-term studies of patients with intracranial stents have not yet been done.
Benefits and risks
At this point it appears that the risks associated with surgical clipping and endovascular coiling, in terms of stroke or death from the procedure, are the same.[8] The ISAT trials have shown, however, that patients who have experienced aneurysmal rupture have a 7% lower mortality rate when treated by coiling than patients treated by clipping, when all other factors are equal. Coiled aneurysms, however, do have a higher recurrence rate as demonstrated by angiography. For instance, the 2007 study by Jacques Moret and colleagues from Paris, France, (a group with one of the largest experiences in endovascular coiling) indicates that 28.6% of aneurysms recurred within one year of coiling, and that the recurrence rate increased with time.[9] These results are similar to those previously reported by other endovascular groups. For instance Jean Raymond and colleagues from Montreal, Canada, (another group with a large experience in endovascular coiling) reported that 33.6% of aneurysms recurred within one year of coiling.[10] The most recent data from Moret's group reveals even higher aneurysm recurrence rates, namely a 36.5% recurrence rate at 9 months (which breaks down as 31.1% for small aneurysms less than 10 mm, and 56.0% for aneurysms 10 mm or larger).[11] However, no studies to date have shown that the higher angiographic recurrence rate equals a higher rate of rebleeding. Thus far, the ISAT trials listed above show no increase in the rate of rebleeding, and show a persistent 7% lower mortality rate in subarachnoid hemorrhage patients who have been treated with coiling. In ISAT, the need for late retreatment of aneurysms was 6.9 times more likely for endovascular coiling as compared to surgical clipping.[12] Furthermore, data from the ISAT group in March 2008 indicates that the higher aneurysm rate of recurrence is associated with a higher rebleeding rate, given that the rebleed rate of coiled aneurysms appears to be 8 times higher than that of surgically treated aneurysms in the ISAT study.[13]
Therefore it appears that although endovascular coiling is associated with a shorter recovery period as compared to surgical clipping, it is also associated with a significantly higher recurrence rate after treatment. The long-term data for unruptured aneurysms are still being gathered.
Patients who undergo endovascular coiling need to have several serial studies (such as MRI/MRA, CTA, or angiography) to detect early recurrences. If a recurrence is identified, the aneurysm may need to be retreated with either surgery or further coiling. The risks associated with surgical clipping of previously-coiled aneurysms are very high. Ultimately, the decision to treat with surgical clipping versus endovascular coiling should be made by a cerebrovascular team with extensive experience in both modalities.
Prognosis
The prognosis for a patient with a ruptured cerebral aneurysm depends on the extent and location of the aneurysm, the person's age, general health, and neurological condition. Some individuals with a ruptured cerebral aneurysm die from the initial bleeding. Other individuals with cerebral aneurysm recover with little or no neurological deficit. The most significant factors in determining outcome are grade (see Hunt and Hess grade above) and age. Generally patients with Hunt and Hess grade I and II hemorrhage on admission to the emergency room and patients who are younger within the typical age range of vulnerability can anticipate a good outcome, without death or permanent disability. Older patients and those with poorer Hunt and Hess grades on admission have a poor prognosis. Generally, about two thirds of patients have a poor outcome, death, or permanent disability.[14][15]
See also
References
- ^ a b c Brisman JL, Song JK, Newell DW (August 2006). "Cerebral aneurysms". N Engl J Med 355 (9): 928–39. doi:10.1056/NEJMra052760. PMID 16943405.
- ^ a b Appel, Jacob M. Health care hard to recognize, tough to define, Albany Times Union, Nov. 12, 2009
- ^ a b What You Should Know About Cerebral Aneurysms, http://www.strokeassociation.org
- ^ Hunt, WE; Hess RM (1968). "Surgical Risk as Related to Time of Intervention in the Repair of Intracranial Aneurysms". J Neurosurg 28 (1): 14–20. doi:10.3171/jns.1968.28.1.0014. PMID 5635959.
- ^ Greenberg, MS (2010). Handbook of Neurosurgery (7th ed). Thieme.
- ^ Gallo, GL; Rafael Tamargo (October 2006). "Leukocyte-endothelial cell interactions in chronic vasospasm after subarachnoid hemorrhage". Neurol. Res 28 (7): 750–8. doi:10.1179/016164106X152025. PMID 17164038.
- ^ http://www.med.unc.edu/neurosurgery/news/germanwala-presents-first-aneurysm-patient-treated-through-nose
- ^ Raja PV, Huang J, Germanwala AV, Gailloud P, Murphy KP, Tamargo RJ (June 2008). "Microsurgical clipping and endovascular coiling of intracranial aneurysms: a critical review of the literature". Neurosurgery 62 (6): 1187–202; discussion 1202–3. doi:10.1227/01.neu.0000333291.67362.0b. PMID 18824986. http://meta.wkhealth.com/pt/pt-core/template-journal/lwwgateway/media/landingpage.htm?issn=0148-396X&volume=62&issue=6&spage=1187.
- ^ Piotin, M; Spelle, L, Mounayer, C, Salles-Rezende, MT, Giansante-Abud, D, Vanzin-Santos, R, Moret, J (May 2007). "Intracranial aneurysms: treatment with bare platinum coils—aneurysm packing, complex coils, and angiographic recurrence". Radiology 243 (2): 500–8. doi:10.1148/radiol.2431060006. PMID 17293572.
- ^ Raymond, J; Guilbert, F, Weill, A, Georganos, SA, Juravsky, L, Lambert, A, Lamoureux, J, Chagnon, M, Roy, D (June 2003). "Long-term angiographic recurrences after selective endovascular treatment of aneurysms with detachable coils". Stroke 34 (6): 1398–1403. doi:10.1161/01.STR.0000073841.88563.E9. PMID 12775880.
- ^ Piotin M, Spelle L, Mounayer C, Loureiros C, Ghorbani A, Moret J (January 2009). "Intracranial aneurysms coiling with matrix: immediate results in 152 patients and midterm anatomic follow-up from 115 patients". Stroke 40 (1): 321–3. doi:10.1161/STROKEAHA.108.520866. PMID 18988913. http://stroke.ahajournals.org/cgi/pmidlookup?view=long&pmid=18988913.
- ^ Campi, A; Ramzi N, Molyneaux AJ, Summers, PE, Kerr, RS, Sneade, M, Yarnold, JA, Rischmiller, J, Byrne, JV (May 2007). "Retreatment of ruptured cerebral aneurysms in patients randomized by coiling or clipping in the International Subarachnoid Aneurysm Trial (ISAT)". Stroke 38 (5): 1538–44. doi:10.1161/STROKEAHA.106.466987. PMID 17395870.
- ^ Mitchell P, Kerr R, Mendelow AD, Molyneux A (March 2008). "Could late rebleeding overturn the superiority of cranial aneurysm coil embolization over clip ligation seen in the International Subarachnoid Aneurysm Trial?". J. Neurosurg. 108 (3): 437–42. doi:10.3171/JNS/2008/108/3/0437. PMID 18312088. http://thejns.org/doi/abs/10.3171/JNS/2008/108/3/0437?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%3dpubmed.
- ^ Hop, Jeanette; Gabriel Rinkel, Ale Algra, Jan van Gijn (March 1997). "Case-Fatality Rates and Functional Outcome after Subarachnoid Hemorrhage: A Systematic Review". Stroke 28 (3): 660–4. doi:10.1161/01.STR.28.3.660. PMID 11157554.
- ^ Ljunggren, B; Sonesson B, Säveland H, Brandt L (1985). "Cognitive impairment and adjustment in patients without neurological deficit after aneurysmal SAH and early operation". Journal of Neurosurgery 62 (5): 673–9. doi:10.3171/jns.1985.62.5.0673. PMID 3989590.
External links
- Aneurysm at the Open Directory Project
- National Institute of Neurological Disorders and Stroke
- Brain Aneurysm & Cerebrovascular Resource
- Cerebral Aneurysm Calculator and Image Library
CNS disease, Vascular disease: Cerebrovascular diseases (G45–G46 and I60–I69, 430–438) Brain ischemia/
cerebral infarction
(ischemic stroke/TIA)Intracranial hemorrhage
(hemorrhagic stroke)Extra-axialBrainstemAneurysm Other/general Cardiovascular disease: vascular disease · Circulatory system pathology (I70–I99, 440–456) Arteries, arterioles
and capillariesAtherosclerosis (Foam cell, Fatty streak, Atheroma, Intermittent claudication) · Monckeberg's arteriosclerosis · Arteriolosclerosis (Hyaline, Hyperplastic, oxycholesterol, cholesterol, LDL, trans fat)Othertorso: Aortic aneurysm (Thoracic aortic aneurysm, Abdominal aortic aneurysm) · Aortic dissection · Coronary artery aneurysmhead/neck: Cerebral aneurysm · Intracranial berry aneurysm · Carotid artery dissection · Vertebral artery dissection · Familial aortic dissectionVeins OtherArteries or veins Blood pressure Hypertensive heart disease · Hypertensive nephropathy · Essential hypertension · Secondary hypertension (Renovascular hypertension) · Pulmonary hypertension · Malignant hypertension · Benign hypertension · Systolic hypertension · White coat hypertensionCategories:- Neurosurgery
- Cerebrovascular diseases
Wikimedia Foundation. 2010.