- Aortic insufficiency
-
See also: mitral regurgitation and tricuspid insufficiency
Aortic insufficiency Classification and external resources 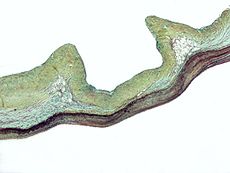
Micrograph of myxomatous degeneration – a cause of aortic insufficiency.ICD-10 I06, I35, Q23.1 ICD-9 395.1, 746.4 DiseasesDB 829 eMedicine med/156 emerg/39 ped/2487 MeSH D001022 Aortic insufficiency (AI), also known as aortic regurgitation (AR), is the leaking of the aortic valve of the heart that causes blood to flow in the reverse direction during ventricular diastole, from the aorta into the left ventricle.[1]
Aortic insufficiency can be due to abnormalities of either the aortic valve or the aortic root (the beginning of the aorta).
Contents
Etiology
About half of the cases of aortic insufficiency are due to the aortic root dilatation (annuloaortic ectasia), which is idiopathic in over 80% of cases, but otherwise may result from aging, syphilitic aortitis, osteogenesis imperfecta, aortic dissection, Behçet's disease, reactive arthritis and systemic hypertension.[2] In about 15% the cause is innate bicuspidal aortic valve, while another 15% cases are due to retraction of the cusps as part of postinflammatory processes of endocarditis in rheumatic fever and various collagen vascular diseases. Additionally, aortic insufficiency has been linked to the use of some medications, specifically medications containing fenfluramine or dexfenfluramine isotopes[3][4] and dopamine agonists.[5][6] Other potential causes that affects the valve directly include: Marfan's syndrome, Ehlers–Danlos syndrome, ankylosing spondylitis and systemic lupus erythematosus.[2] In acute cases of aortic insufficiency, the main causes are infective endocarditis[2][7] or trauma.[2]
Physiology
In individuals with a normally functioning aortic valve, the valve is only open when the pressure in the left ventricle is higher than the pressure in the aorta. This allows the blood to be ejected from the left ventricle into the aorta during ventricular systole. The amount of blood that is ejected by the heart is known as the stroke volume. Under normal conditions, 50–70% of the blood in a filled left ventricle is ejected into the aorta to be used by the body (called the 'ejection fraction'). After ventricular systole, the pressure in the left ventricle decreases as it relaxes and begins to fill up with blood from the left atrium. This relaxation of the left ventricle (early ventricular diastole) causes a fall in its pressure. When the pressure in the left ventricle falls below the pressure in the aorta, the aortic valve will close, preventing blood in the aorta from going back into the left ventricle.
Pathophysiology
In aortic insufficiency (AI), when the pressure in the left ventricle falls below the pressure in the aorta, the aortic valve is not able to completely close. This causes a leaking of blood from the aorta into the left ventricle. This means that some of the blood that was already ejected from the heart is regurgitating back into the heart. The percentage of blood that regurgitates back through the aortic valve due to AI is known as the regurgitant fraction. For instance, if an individual with AI has a stroke volume of 100 ml and during ventricular diastole 25 ml regurgitates back through the aortic valve, the regurgitant fraction is 25%. This regurgitant flow causes a decrease in the diastolic blood pressure in the aorta, and therefore an increase in the pulse pressure (systolic pressure - diastolic pressure). Thus, physical examination will reveal a bounding pulse, especially in the radial artery.
Since some of the blood that is ejected during systole regurgitates back into the left ventricle during diastole, there is decreased effective forward flow in AI.
Note that while diastolic blood pressure is diminished and the pulse pressure widens, systolic blood pressure generally remains normal or can even be slightly elevated. This is because sympathetic nervous system and the renin-angiotensin-aldosterone axis of the kidneys compensate for the decreased cardiac output. Catecholamines will increase the heart rate and increase the strength of ventricular contraction, directly increasing cardiac output. Catecholamines will also cause peripheral vasoconstriction, which causes increased systemic vascular resistance and ensures that core organs are adequately perfused. Renin, a proteolytic enzyme, cleaves angiotensinogen to angiotensin I, which is converted to angiotensin II, which is also a potent vasoconstrictor. In the case of chronic aortic insufficiency with resultant cardiac remodeling, heart failure will develop, and it is possible to see systolic pressures diminish.
Aortic insufficiency causes both volume overload (elevated preload) and pressure overload (elevated afterload due to increased stroke volume) of the heart.
The pressure overload (due to elevated pulse pressure and the systemic effects of neuroendocrine hormones) causes left ventricular hypertrophy (LVH). There is both concentric hypertrophy and eccentric hypertrophy in AI. The concentric hypertrophy is due to the increased left ventricular systolic pressures associated with AI, while the eccentric hypertrophy is due to volume overload caused by the regurgitant fraction.
Hemodynamics
The hemodynamic sequelae of AI are dependent on the rate of onset of AI. Acute AI and chronic AI will have different hemodynamics and individuals will have different signs and symptoms.
Acute aortic insufficiency
In acute AI, as may be seen with acute perforation of the aortic valve due to endocarditis, there will be a sudden increase in the volume of blood in the left ventricle. The ventricle is unable to deal with the sudden change in volume. In terms of the Frank-Starling curve, the end-diastolic volume will be very high, such that further increases in volume result in less and less efficient contraction. The filling pressure of the left ventricle will increase. This causes pressure in the left atrium to rise, and the individual will develop pulmonary edema.
Severe acute aortic insufficiency is considered a medical emergency. There is a high mortality rate if the individual does not undergo immediate surgery for aortic valve replacement. If the acute AI is due to aortic valve endocarditis, there is a risk that the new valve may become seeded with bacteria. However, this risk is small.[8]
Acute AI usually presents as florid congestive heart failure, and will not have any of the signs associated with chronic AI since the left ventricle had not yet developed the eccentric hypertrophy and dilatation that allow an increased stroke volume, which in turn cause bounding peripheral pulses. On auscultation, there may be a short diastolic murmur and a soft S1. S1 is soft because the elevated filling pressures close the mitral valve in diastole (rather than the mitral valve being closed at the beginning of systole).
Chronic aortic insufficiency
If the individual survives the initial hemodynamic derailment that acute AI presents as, the left ventricle adapts by eccentric hypertrophy and dilatation of the left ventricle, and the volume overload is compensated for. The left ventricular filling pressures will revert to normal and the individual will no longer have overt heart failure.
In this compensated phase, the individual may be totally asymptomatic and may have normal exercise tolerance.
Eventually (typically after a latency period) the left ventricle will become decompensated, and filling pressures will increase. While most individuals would complain of symptoms of congestive heart failure to their physicians, some enter this decompensated phase asymptomatically. Proper treatment for AI involves aortic valve replacement prior to this decompensation phase.
Symptoms
Symptoms of aortic insufficiency are similar to those of heart failure and include dyspnea on exertion, orthopnea and paroxysmal nocturnal dyspnea.[2] Palpitations and angina pectoris may also be felt.[2] In acute cases there may be cyanosis and circulatory shock.[2]
Physical examination
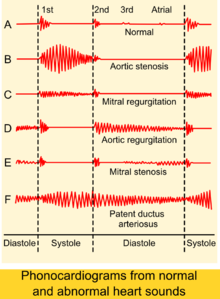
The physical examination of an individual with aortic insufficiency involves auscultation of the heart to listen for the murmur of aortic insufficiency and the S3 heart sound (S3 gallop correlates with development of LV dysfunction).[2] The murmur of chronic aortic insufficiency is typically described as early diastolic and decrescendo, which is best heard at aortic area when the patient is seated and leans forward with breath held in expiration. The murmur is usually soft and seldom causes thrill. there is radiation to the right parasternal region, ascending aortic aneurysm has to be excluded. The apex beat is typically displaced down and to the left[2]
If there is increased stroke volume of the left ventricle due to volume overload, an ejection systolic 'flow' murmur may also be present when auscultating the same aortic area. Unless there is concomitant aortic valve stenosis, the murmur should not start with an ejection click.
There may also be an Austin Flint murmur,[2] a soft mid-diastolic rumble heard at the apical area. It appears when regurgitant jet from the severe aortic insufficiency renders partial closure of the anterior mitral leaflet.
Peripheral physical signs of aortic insufficiency are related to the high pulse pressure and the rapid decrease in blood pressure during diastole due to blood returning to the heart (the wrong way) from the aorta through the incompetent aortic valve, although the usefulness of some of the eponymous signs has been questioned:[9]
- large-volume, 'collapsing' pulse also known as:
- Watson's water hammer pulse
- Corrigan's pulse (rapid upstroke and collapse of the carotid artery pulse)
- low diastolic and increased pulse pressure
- de Musset's sign (head nodding in time with the heart beat)
- Quincke's sign (pulsation of the capillary bed in the nail; named for Heinrich Quincke)
- Traube's sign (a 'pistol shot' systolic sound heard over the femoral artery; named for Ludwig Traube)
- Duroziez's sign (systolic and diastolic murmurs heard over the femoral artery when it is gradually compressed with the stethescope)
Also, these are usually less detectable in acute cases.[7]
Less used signs include:[10]
- Lighthouse sign (blanching & flushing of forehead)
- Landolfi's sign (alternating constriction & dilatation of pupil)
- Becker's sign (pulsations of retinal vessels)
- Müller's sign (pulsations of uvula)
- Mayen's sign (diastolic drop of BP>15 mm Hg with arm raised)
- Rosenbach's sign (pulsatile liver)
- Gerhardt's sign (enlarged spleen)
- Hill's sign - a ≥ 20 mmHg difference in popliteal and brachial systolic cuff pressures, seen in chronic severe AI. Considered to be an artefact of sphygmomanometric lower limb pressure measurement.[11]
- Lincoln sign (pulsatile popliteal)
- Sherman sign (dorsalis pedis pulse is quickly located & unexpectedly prominent in age>75 yr)
- Ashrafian sign (Pulsatile pseudo-proptosis)[10]
Unfortunately, none of the above putative signs of aortic insufficiency is of utility in making the diagnosis,[12] but they may help as pointers. What is of value is hearing a diastolic murmur itself, whether or not the above signs are present.
Diagnostic evaluation
The most common test used for the evaluation of the severity of aortic insufficiency is transthoracic echocardiography, which can provide two-dimensional views of the regurgitant jet, allow measurement of velocity using Doppler, and estimate jet volume.
The findings in severe aortic regurgitation, based on the 2006 American College of Cardiology/American Heart Association guidelines include:
- An AI color jet width > 65 percent of the left ventricular outflow tract (LVOT) diameter (may not be true if the jet is eccentric)
- Doppler vena contracta width > 0.6 cm
- The pressure half-time of the regurgitant jet is < 250 msec
- Early termination of the mitral inflow (due to increase in LV pressure due to the AI.)
- Holodiastolic flow reversal in the descending aorta.
- Regurgitant volume > 60 ml
- Regurgitant fraction > 50 percent
- Regurgitant orifice area > 0.3 cm2
- Increased left ventricular size
In acute aortic regurgitation, echocardiography may show early closure of the mitral valve.[2]
Chest X-ray can assist in making the diagnosis, showing left ventricular hypertrophy and dilated aorta.[2] ECG typically indicates left ventricular hypertrophy.[2] Cardiac chamber catetherization assists in assessing the severity of regurgitation and any left ventricular dysfunction.[2]
Prognosis
The risk of death in individuals with aortic insufficiency, dilated ventricle, normal ejection fraction who are asymptomatic is about 0.2 percent[citation needed] per year. Risk increases if the ejection fraction decreases or if the individual develops symptoms.
Treatment
Indications for surgery for chronic severe aortic insufficiency[13] Symptoms Ejection fraction Additional Findings Present
(NYHA II[7]-IV)Any Absent > 50 % Abnormal exercise test, severe LV dilatation
(systolic ventricular diameter >55 mm[7])Absent <=50 % Cardiac surgery for other cause (i.e.: CAD, other valvular disease, ascending aortic aneurysm)
Aortic insufficiency can be treated either medically or surgically, depending on the acuteness of presentation, the symptoms and signs associated with the disease process, and the degree of left ventricular dysfunction.Surgical treatment is controversial in asymptomatic patients, however has been recommended if the ejection fraction falls to 50% or below, in the face of progressive and severe left ventricular dilatation, or with symptoms or abnormal response to exercise testing. For both groups of patients, surgery before the development of worsening ejection fraction/LV dilatation, is expected to reduce the risk of sudden death, and is associated with lower peri-operative mortality. Also, surgery is optimally performed immediately in acute cases.[2]
Medical treatment
Medical therapy of chronic aortic insufficiency that is stable and asymptomatic involves the use of vasodilators.[2] Small trials have shown a short term benefit in the use of ACE inhibitors or angiotensin II receptor antagonists, nifedipine, and hydralazine in improving left ventricular wall stress, ejection fraction, and mass. The use of these vasodilators is only indicated in individuals who suffer from hypertension in addition to AI. The goal in using these pharmacologic agents is to decrease the afterload so that the left ventricle is somewhat spared. The regurgitant fraction may not change significantly, since the gradient between the aortic and left ventricular pressures is usually fairly low at the initiation of treatment.
Other rather conservative medical treatments for stable and asymptomatic cases include low sodium diet,[2] diuretics,[2] digoxin,[2] calcium blockers[7] and avoiding very strenuous activity.[2][7]
In addition, endocarditis prophylaxis is indicated before dental, gastrointestinal or genitourinary procedures.[2]
In mild to moderate cases, echocardiography and cardiac stress test should be followed up every 1–2 years. In severe moderate/severe cases, echocardiography with cardiac stress test and/or isotope perfusion imaging should be performed every 3–6 months.[7]
Surgical treatment
The surgical treatment of choice at this time is an aortic valve replacement. This is currently an open-heart procedure, requiring the individual to be placed on cardiopulmonary bypass.
In the case of severe acute aortic insufficiency, all individuals should undergo surgery if there are no absolute contraindications for surgery. Individuals with bacteremia with aortic valve endocarditis should not wait for treatment with antibiotics to take effect, given the high mortality associated with the acute AI. Instead, replacement with an aortic valve homograft should be performed if feasible.
A percutaneous approach to aortic valve replacement is now feasible, but the main experience has been in the treatment of aortic stenosis.
References
- ^ Aortic regurgitation at Mount Sinai Hospital
- ^ a b c d e f g h i j k l m n o p q r s t u Chapter 1: Diseases of the Cardiovascular system > Section: Valvular Heart Disease in: Elizabeth D Agabegi; Agabegi, Steven S. (2008). Step-Up to Medicine (Step-Up Series). Hagerstwon, MD: Lippincott Williams & Wilkins. ISBN 0-7817-7153-6.
- ^ Connolly HM, Crary JL, McGoon MD et al. (1997). "Valvular heart disease associated with fenfluramine-phentermine". N. Engl. J. Med. 337 (9): 581–8. doi:10.1056/NEJM199708283370901. PMID 9271479. http://content.nejm.org/cgi/content/full/337/9/581.
- ^ Weissman NJ (2001). "Appetite suppressants and valvular heart disease". Am. J. Med. Sci. 321 (4): 285–91. doi:10.1097/00000441-200104000-00008. PMID 11307869.
- ^ Schade R, Andersohn F, Suissa S, Haverkamp W, Garbe E (2007). "Dopamine agonists and the risk of cardiac-valve regurgitation". N. Engl. J. Med. 356 (1): 29–38. doi:10.1056/NEJMoa062222. PMID 17202453.
- ^ Zanettini R, Antonini A, Gatto G, Gentile R, Tesei S, Pezzoli G (2007). "Valvular heart disease and the use of dopamine agonists for Parkinson's disease". N. Engl. J. Med. 356 (1): 39–46. doi:10.1056/NEJMoa054830. PMID 17202454.
- ^ a b c d e f g VOC=VITIUM ORGANICUM CORDIS, a compendium of the Department of Cardiology at Uppsala Academic Hospital. By Per Kvidal September 1999, with revision by Erik Björklund May 2008
- ^ al Jubair K, al Fagih MR, Ashmeg A, Belhaj M, Sawyer W (1992). "Cardiac operations during active endocarditis". J. Thorac. Cardiovasc. Surg. 104 (2): 487–90. PMID 1495315.
- ^ Babu AN, Kymes SM, Carpenter Fryer SM (2003). "Eponyms and the diagnosis of aortic regurgitation: what says the evidence?". Ann. Intern. Med. 138 (9): 736–42. PMID 12729428.
- ^ a b Ashrafian H. Pulsatile pseudo-proptosis, aortic regurgitation and 31 eponyms. Int J Cardiol. 2006 Mar 8;107(3):421-3.
- ^ Kutryk M, Fitchett D (1997). "Hill's sign in aortic regurgitation: enhanced pressure wave transmission or artefact?". The Canadian journal of cardiology 13 (3): 237–40. PMID 9117911.
- ^ Choudhry NK, Etchells EE (1999). "The rational clinical examination. Does this patient have aortic regurgitation?". JAMA 281 (23): 2231–8. PMID 10376577.
- ^ Bonow, RO; American Heart Association Task Force on Practice Guidelines (Writing Committee to revise the 1998 guidelines for the management of patients with valvular heart disease); Society of Cardiovascular Anesthesiologists; Bonow, RO; Carabello, BA; Chatterjee, K; De Leon Jr, AC; Faxon, DP et al. (2006). "ACC/AHA guidelines for the management of patients with valvular heart disease. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines". J. Am. Coll. Cardiol. 48 (3): e1–148. doi:10.1016/j.jacc.2006.05.021. PMID 16875962.
External links
- Heart Disease and Life After by Richard Hinkle
See also
Congenital heart defects (Q20–Q24, 745–746) Cardiac shunt/
heart septal defectAortopulmonary septal defectR→L: Double outlet right ventricle (Taussig–Bing syndrome) · Transposition of the great vessels (dextro, levo) · Persistent truncus arteriosusL→R: Sinus venosus atrial septal defect · Lutembacher's syndromeL→R and R→L: Eisenmenger's syndromeR→L, with other conditions: Tetralogy of FallotL→R: Ostium primumValvular heart disease/
heart chambersRightpulmonary valves (stenosis, insufficiency) · tricuspid valves (stenosis, atresia, Ebstein's anomaly) · Hypoplastic right heart syndrome (Uhl anomaly)Leftaortic valves (stenosis, insufficiency, bicuspid) · mitral valves (stenosis, regurgitation) · Hypoplastic left heart syndromeOther Dextrocardia · Levocardia · Cor triatriatum · Crisscross heart · Brugada syndrome · Coronary artery anomaly · Anomalous aortic origin of a coronary artery · Ventricular inversionCategories: - large-volume, 'collapsing' pulse also known as:
Wikimedia Foundation. 2010.