- Meningococcal disease
-
Meningococcal disease Classification and external resources 
Charlotte Cleverley-Bisman, one of the youngest survivors of the disease. The infected arms had to be amputated later.ICD-10 A39 ICD-9 036.9 DiseasesDB 8847 MedlinePlus 000608 meningitis. 001349 meningococcemia Meningococcal disease describes infections caused by the bacterium Neisseria meningitidis (also termed meningococcus). It carries a high mortality rate if untreated. While best known as a cause of meningitis, widespread blood infection (sepsis) is more damaging and dangerous. Meningitis and Meningococcemia are major causes of illness, death, and disability in both developed and under developed countries worldwide.
The disease's host/pathogen interaction is not fully understood. The pathogen originates harmlessly in a large number of the general population, but thereafter can invade the blood stream and the brain, causing serious illness. Over the past few years, experts have made an intensive effort to understand specific aspects of meningococcal biology and host interactions, however the development of improved treatments and effective vaccines will depend on novel efforts by workers in many different fields.[1]
The incidence of endemic meningococcal disease during the last 13 years ranges from 1 to 5 per 100,000 in developed countries, and from 10 to 25 per 100,000 in developing countries. During epidemics the incidence of meningococcal disease approaches 100 per 100,000. There are approximately 2,600 cases of bacterial meningitis per year in the United States, and on average 333,000 cases in developing countries. The case fatality rate ranges between 10 and 20 per cent.[2]
While Meningococcal disease is not as contagious as the common cold (which is spread through casual contact), it can be transmitted through saliva and occasionally through close, prolonged general contact with an infected person.
Pathogenesis
Meningococcal disease causes life-threatening meningitis and sepsis conditions. In the case of meningitis, bacteria attack the lining between the brain and skull called the meninges. Infected fluid from the meninges then passes into the spinal cord, causing symptoms including stiff neck, fever and rashes. The meninges (and sometimes the brain itself) begin to swell, which affects the central nervous system.
Even with antibiotics, approximately 1 in 10 victims of meningococcal meningitis will die; However, about as many survivors of the disease lose a limb or their hearing, or suffer permanent brain damage.[3] The sepsis type of infection is much more deadly, and results in a severe blood poisoning called meningococcal sepsis that affects the entire body. In this case, bacterial toxins rupture blood vessels and can rapidly shut down vital organs. Within hours, patient's health can change from seemingly good to mortally ill.[4]
The N. meningitidis bacterium is surrounded by a slimy outer coat that contains disease-causing endotoxin. While many bacteria produce endotoxin, the levels produced by meningococcal bacteria are 100 to 1,000 times greater (and accordingly more lethal) than normal. As the bacteria multiply and move through the bloodstream, it sheds concentrated amounts of toxin. The endotoxin directly affects the heart, reducing its ability to circulate blood, and also causes pressure on blood vessels throughout the body. As some blood vessels start to hemorrhage, major organs like the lungs and kidneys are damaged.
Patients suffering from meningococcal disease are treated with a large dose of antibiotic. The systemic antibiotic flowing through the bloodstream rapidly kills the bacteria but, as the bacteria are killed, even more toxin is released. It takes up to several days for the toxin to be neutralized from the body by using continuous liquid treatment and antibiotic therapy.[5]
Meningococcal is often spread through saliva. This can be from kissing or drinking from someone else's cup
Meningitis
The patient with meningococcal meningitis typically presents with high fever, meningism (stiff neck), Kernig's sign, severe headache, vomiting, purpura, photophobia, and sometimes chills, altered mental status, or seizures. Diarrhea or respiratory symptoms are less common. Petechiae is often also present, but does not always occur, so its absence should not be used against the diagnosis of meningococcal disease. Anyone with symptoms of meningococcal meningitis should receive intravenus antibiotics pending results of lumbar puncture, as delay in treatment worsens the prognosis.
Meningococcemia
Symptoms of meningococcemia are, at least initially, similar to those of influenza. Typically, the first symptoms include fever, nausea, myalgia, headache, arthralgia, chills, diarrhea, stiff neck, and malaise. Later symptoms include septic shock, purpura, hypotension, cyanosis, petechiae, seizures, anxiety, and multiple organ dysfunction syndrome. Acute respiratory distress syndrome and altered mental status may also occur. Meningococcal sepsis has a higher mortality rate than meningococcal meningitis, but the risk of neurologic sequelae is much lower.[citation needed]
Types of infection
Meningococcemia
Meningococcemia, like many gram-negative blood infections, can cause disseminated intravascular coagulation (DIC), a condition where blood starts to clot throughout the body, sometimes causing ischemic tissue damage. DIC also causes bleeding, when the clotting factors are used up, causing the characteristic purpuric rash.
Meningitis
Meningococcal meningitis is a consequence of bacteria entering the cerebrospinal fluid (CSF) and irritating the meninges - the membranes that line the brain and spinal cord. Sub-Saharan Africa, Americas, Western Europe, UK and Ireland face multifarious challenges, 200 years after the discovery of bacterial meningitis.[6]
Other types
As with any gram negative bacterium, N. meningitidis can infect a variety of sites.
Meningococcal pneumonia can appear during influenza pandemics and in military camps. This is a multilobar, rapidly evolving pneumonia, sometimes associated with septic shock. With prompt treatment with penicillin or chloramphenicol, the prognosis is excellent. Pericarditis can appear, either as a septic pericarditis with grave prognosis or as a rective pericarditis in the wake of meningitis or septicaemia. Myocarditis can be a complication of meningococcemia and can be contributive to shock seen in this form of disease. Pharyngitis and conjunctivitis can also appear and can constitute the portal of entry for the bacterium. Septic arthritis due to N. meningitidis can be seen, usually accompanying disseminated infection. Other forms of disease can rarely be seen, like osteomyelitis, endophthalmitis and urethritis.
Meningococcal disease in Africa
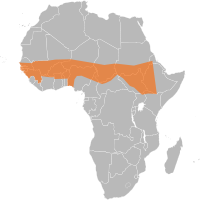
The importance of meningitis disease is as significant in Africa as HIV, TB and Malaria. Cases of meningococcal disease due to Neisseria meningitidis meningococcaemia leading to severe meningoencephalitis are common among young children and the elderly. Deaths occurring in less than 24-hours are more likely during the disease epidemic seasons in Africa and Sub-Saharan Africa is hit by meningitis disease outbreaks throughout the epidemic season. Climate change[7] contributes significantly the spread of the disease in Benin, Burkina Faso, Cameroon, the Central African Republic, Chad, Côte d'Ivoire, the Democratic Republic of the Congo, Ethiopia, Ghana, Mali, Niger, Nigeria and Togo. This is an area of Africa where the disease is endemic: meningitis is "silently" present, and there are always a few cases. When the number of cases passes five per population of 100,000 in one week, teams are on alert. Epidemic levels are reached when there have been 100 cases per 100,000 populations over several weeks.[8] Further complicating efforts to halt the spread of meningitis in Africa is the fact that extremely dry, dusty weather conditions which characterize Niger and Burkina Faso from December to June favor the development of epidemics. Overcrowded villages are breeding grounds for bacterial transmission and lead to a high prevalence of respiratory tract infections, which leave the body more susceptible to infection, encouraging the spread of meningitis. IRIN Africa news has been providing the number of deaths for each country since 1995,[9][10][11][12] and a mass vaccination campaign following a community outbreak of Meningococcal disease in Florida was done by the CDC.[13]
Prevention
The most important form of prevention is a vaccine against N. meningitidis. Different countries have different strains of the bacteria and therefore use different vaccines. Five serogroups, A, B, C, Y and W135 are responsible for virtually all cases of the disease in humans. Vaccines are currently available against four of the five strains, and a vaccine against the B strain is in development.[14] Menveo of Novartis vaccines Menactra, Menomune of Sanofi-Aventis, Mencevax of GlaxoSmithKline and NmVac4-A/C/Y/W-135 (has not been licensed in the US) of JN-International Medical Corporation are the commonly used vaccines. Vaccines offer significant protection from three to five years (plain polysaccharide vaccine Menomune, Mencevax and NmVac-4) to more than eight years (conjugate vaccine Menactra).[15][16]
Vaccinations
Children
Children 2–10 years of age who are at high risk for meningococcal disease such as certain chronic medical conditions and travel to or reside in countries with hyperendemic or epidemic meningococcal disease should receive primary immunization. Although safety and efficacy of the vaccine have not been established in children younger than 2 years of age and under outbreak control, the unconjugated vaccine can be considered....[17][18][19][20]
Children and adolescents 11 years of age or older
It is recommended that primary immunization against meningococcal disease with Meningitis A,C,Y and W-135 vaccines for all young adolescents at 11–12 years of age and all unvaccinated older adolescents at 15 years of age. Although conjugate vaccines are the preferred meningococcal vaccine in adolescents 11 years of age or older, polysaccharide vaccines are an acceptable alternative if the conjugated vaccine is unavailable.[18][19][21][21]
Adults
College Students who plan to live in dormitories receive primary immunization with Meningitis A, C, Y and W-135 vaccines, although the risk for meningococcal disease for co-eds 18–24 years of age is similar to that of the general population of similar age. College students consider vaccination against meningococcal disease to reduce their risk for the disease and state that college healthcare providers should take a proactive role in providing information about meningococcal disease to students and their parents.[22] Routine primary immunization against meningococcal disease is recommended for most adults living in endemic areas or planning to travel to such areas. Although conjugate vaccines are the preferred meningococcal vaccine in adults 55 years of age or younger, polysaccharide vaccines are an acceptable alternative for adults in this age group if the conjugated vaccine is unavailable. Since safety and efficacy of conjugate vaccines in adults older than 55 years of age have not been established to date, polysaccharide vaccines should be used for primary immunization in this group.[18][19]
Medical staff and laboratory personnel
Health care people should receive routine immunization against meningococcal disease for laboratory personnel who are routinely exposed to isolates of N. meningitidis. Laboratory personnel and medical staff are at risk of exposure to N. meningitides or to patients with meningococcal disease. Hospital Infection Control Practices Advisory Committee (HICPAC) recommendations regarding immunization of health-care workers that routine vaccination of health-care personnel is recommended, Any individual 11–55 years of age who wishes to reduce their risk of meningococcal disease may receive Meningitis A,C,Y and W-135 vaccines and those older than 55 years of age. Under certain circumstances if unvaccinated health-care personnel cannot get vaccinated and who have intensive contact with oropharyngeal secretions of infected patients and who do not use proper precautions should receive anti-infective prophylaxis against meningococcal infection (i.e., 2-day regimen of oral rifampin or a single dose of IM ceftriaxone or a single dose of oral ciprofloxacin).[18][23]
Military recruits
Because the risk of meningococcal disease is increased among military recruits, all military recruits routinely receive primary immunization against the disease.[24]
Travelers and tourists
Immunization against meningococcal disease is not a requirement for entry into any country, unlike Yellow fever. Only Saudi Arabia require that travelers to their country for the annual Hajj and Umrah pilgrimage have a certificate of vaccination against meningococcal disease issued not more than 3 years and not less than 10 days before arrival in Saudi Arabia.
Travelers to or residents of areas where N. meningitidis is highly endemic or epidemic are at risk of exposure should receive primary immunization against meningococcal disease.[19][25]
HIV-infected individuals
HIV-infected individuals are likely to be at increased risk for meningococcal disease; HIV-infected individuals who wish to reduce their risk of meningococcal disease may receive primary immunization against meningococcal disease.[23] Although efficacy of Meningitis A,C,Y and W-135 vaccines have not been evaluated in HIV-infected individuals to date, HIV-infected individuals 11–55 years of age may receive primary immunization with the conjugated vaccine.[23] Vaccination against meningitis do not decrease CD4+ T-cell counts or increase viral load in HIV-infected individuals and there has been no evidence that the vaccines adversely affect survival.[26][27][28]
Household and other close contacts of individuals with invasive meningococcal disease
Protective levels of anticapsular antibodies are not achieved until 7–14 days following administration of a meningococcal vaccine, vaccination cannot prevent early onset disease in these contacts and usually is not recommended following sporadic cases of invasive meningococcal disease. Unlike developed countries, in sub-Saharan Africa and other under developed countries, entire families live in a single room of a house.[29][30] Meningococcal infection is usually introduced into a household by an asymptomatic person. Carriage then spreads through the household, reaching infants usually after one or more other household members have been infected. Disease is most likely to occur in infants and young children who lack immunity to the strain of organism circulating and who subsequently acquire carriage of an invasive strain.[31] By preventing susceptible contacts from acquiring infection by directly inhibiting colonization. Close contacts are defined as those persons who could have had intimate contact with the patient’s oral secretions such as through kissing or sharing of food or drink. The importance of the carrier state in meningococcal disease is well known. In developed countries the disease transmission usually occurs in day care, schools and large gatherings where usually disease transmission could occur. Because the meningococcal organism is transmitted by respiratory droplets and is susceptible to drying, it has been postulated that close contact is necessary for transmission. Therefore, the disease transmission to other susceptible person cannot be prevented. Meningitis occurs sporadically throughout the year, and since the organism has no known reservoir outside of man, asymptomatic carriers are usually the source of transmission.[32] Additionally, basic hygiene measures, such as handwashing and not sharing drinking cups, can reduce the incidence of infection by limiting exposure. When a case is confirmed, all close contacts with the infected person can be offered antibiotics to reduce the likelihood of the infection spreading to other people. However, rifampin-resistant strains have been reported and the indiscriminate use of antibiotics contributes to this problem. Chemoprophylaxis is commonly used to those close contacts who are at highest risk of carrying the pathogenic strains. Vaccinations are the only answer for reducing the transmission of the Meningococcal disease.[33][34]
Treatment and prognosis
When meningococcal disease is suspected, treatment must be started immediately and should not be delayed while waiting for investigations. Treatment in primary care usually involves prompt intramuscular administration of benzylpenicillin, and then an urgent transfer to hospital for further care. Once in hospital, the antibiotics of choice are usually IV broad spectrum 3rd generation cephalosporins, e.g. cefotaxime or ceftriaxone. Benzylpenicillin and chloramphenicol are also effective. Supportive measures include IV fluids, oxygen, inotropic support, e.g. dopamine or dobutamine and management of raised intracranial pressure. Steroid therapy may help in some adult patients, but is unlikely to affect long term outcomes.
Complications following meningococcal disease can be divided into early and late groups. Early complications include: raised intracranial pressure, disseminated intravascular coagulation, seizures, circulatory collapse and organ failure. Later complications are: deafness, blindness, lasting neurological deficits, reduced IQ, and gangrene leading to amputations.
Disease Outbreak Control
Meningitis A,C,Y and W-135 vaccines can be used for large-scale vaccination programs when an outbreak of meningococcal disease occurs in Africa and other regions of the world. Whenever sporadic or cluster cases or outbreaks of meningococcal disease occur in the US, chemoprophylaxis is the principal means of preventing secondary cases in household and other close contacts of individuals with invasive disease. Meningitis A,C,Y and W-135 vaccines rarely may be used as an adjunct to chemoprophylaxis,1 but only in situations where there is an ongoing risk of exposure (e.g., when cluster cases or outbreaks occur) and when a serogroup contained in the vaccine is involved. It is important that clinicians promptly report all cases of suspected or confirmed meningococcal disease to local public health authorities and that the serogroup of the meningococcal strain involved be identified. The effectiveness of mass vaccination programs depends on early and accurate recognition of outbreaks. When a suspected outbreak of meningococcal disease occurs, public health authorities will then determine whether mass vaccinations (with or without mass chemoprophylaxis) is indicated and delineate the target population to be vaccinated based on risk assessment.[18]
Meningococcal vaccination and people with chronic medical conditions
Persons with component deficiencies in the final common complement pathway (C3,C5-C9) are more susceptible to N. meningitidis infection than complement-satisfactory persons,[35][36][37][38][39][40][41] and it was estimated that the risk of infection is 7000 times higher in such individuals.[42] In addition, complement component-deficient population frequently experience frequent meningococcal disease[43] since their immune response to natural infection may be less complete than that of complement none-deficient persons.[44][45] Inherited properdin deficiency also is related with an increased risk of contracting meningococcal disease.[46][47] Because persons with functional or anatomic asplenia may not immune to efficiently clear encapsulated Neisseria meningitidis from the bloodstream[48][49] Persons with other conditions associated with immunosuppression also may be at increased risk of developing meningitis disease.[50][51]
Gallery
See also
References
- ^ Meningococcal Disease (2001) Humana Press, Andrew J. Pollard and Martin C.J. Maiden
- ^ Reido, F.S. et al. 1995. Ped. Infect. Dis. J. 14, PP. 643–657
- ^ Centers for Disease Control and Prevention. Meningococcal disease among college students: ACIP modifies recommendations for meningitis vaccination. Press release. 1999 Oct 20
- ^ Jeeri R. Reddy and Thiombiano S. Rigobert. Infections à méningocoques Maladies infectieuses et Africa. West Africa. Med. Bull. 2007
- ^ Jeeri R Reddy and Thiombiano S. Rigobert. Infections à méningocoques Maladies infectieuses et Africa. West Africa. Med. Bull. 2008
- ^ WHO/EMC/BAC/98.3
- ^ AFRICA: Climate change linked to spread of disease
- ^ Meningococcal meningitis
- ^ BURKINA FASO: Meningitis kills more than 400
- ^ Less Vaccine Can Be More: SCIENCE. VOL 322, 5 DECEMBER 2008. Page 1449
- ^ BURKINA FASO: 5 million at risk as meningitis death toll climbs
- ^ NIGER: Nearly 1,000 deaths from meningitis
- ^ Meningococcal Disease: Frequently Asked Questions
- ^ Novartis Phase III study shows meningococcal B vaccine candidate could be first to provide broad coverage against deadly disease
- ^ Conjugate Meningococcal Vaccine Benefits
- ^ Mass Vaccination Campaign Following Community Outbreak of Meningococcal Disease in Florida [1]
- ^ Trotter CL, Andrews NJ, Kaczmarski EB et al. Effectiveness of meningococcal serogroup C conjugate vaccine 4 years after introduction. Lancet. 2004; 364:365-7
- ^ a b c d e Centers for Disease Control and Prevention. Prevention and control of meningococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2005;54(No. RR-7):1-21
- ^ a b c d American Academy of Pediatrics Committee on Infectious Diseases. Policy statement on the prevention and control of meningococcal disease: recommendations for use of meningococcal vaccines in pediatric patients. Pediatrics. 2005;116:496-505
- ^ Pichichero M, Casey J, Blatter M et al. Comparative trial of the safety and immunogenicity of quadrivalent (A, C, Y, W-135) meningococcal polysaccharide-diphtheria conjugate vaccine versus quadrivalent polysaccharide vaccine in two- to ten-year-old children. Pediatr Infect Dis. 2005; 24:57-62
- ^ a b Centers for Disease Control and Prevention Advisory Committee on Immunization Practices, American Academy of Pediatrics, and American Academy of Family Physicians. Recommended childhood and adolescent immunization schedule–United States, 2006. Pediatrics. 2006
- ^ Centers for Disease Control and Prevention. Meningococcal disease among college students: ACIP modifies recommendations for meningitis vaccination. Press release. 1999 Oct 20
- ^ a b c Centers for Disease Control and Prevention. Immunization of health-care workers: recommendations of the Advisory Committee on Immunization Practices (ACIP) and the Hospital Infection Control Practices Advisory Committee (HICPAC). MMWR Morb Mortal Wkly Rep. 1997; 46(No. RR-18):1-42
- ^ Centers for Disease Control and Prevention. Prevention and control of meningococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2005;54 (No. RR-7):1-21. US military recruits should receive routine vaccinations while in service in endemic disease areas
- ^ Centers for Disease Control and Prevention. Prevention and control of meningococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2005;54 (No. RR-7):1-21
- ^ Janoff EN, Tasker S, Opstad NL et al. Impact of immunization of recent HIV-1 seroconverters. Proceedings of ICAAC New Orleans 1996. Abstract No. I60
- ^ Kroon FP, Bruisten S, Swieten PV et al. No increase in HIV-load following immunization with conjugate pneumococcal vaccine, Pneumovax, or Typhim-Vi. Proceedings of ICAAC New Orleans 1996. Abstract No. I61
- ^ Tasker SA, Treanor J, Rossetti R et al. Whole virion influenza vaccine has protective efficacy in the setting of HIV infection. Proceedings of ICAAC New Orleans 1996. Abstract No. I88
- ^ Obaro S. Control of meningococcal disease in west Africa. Lancet 2000;355:1184-B
- ^ Akpede GO. Presentation and outcome of sporadic acute bacterial meningitis in children in the African meningitis belt: recent experience from Northern Nigeria highlighting emergent factors in outcome. West African Journal of Medicine 1995;14:217- 26
- ^ Munford RS, Taunay AE, Morais JS, Fraser DW, Feldman RA. Spread of meningococcal infection within households. Lancet, 1974;ii:1275-78
- ^ Control and Prevention of Meningococcal Disease:Recommendations of the Advisory Committee on Immunization Practices (ACIP): VIRGINIA EPIDEMIOLOGY BULLETIN, July 1997, Volume 97, Number 7
- ^ Jeeri R. Reddy, Safety and Immunogenicity of Meningococcal Meningitis Quadrivalent (A,C,Y & W-135) Polysaccharide Vaccine "PHASE III MULTICENTER CLINICAL TRIAL IN SUB-SAHARAN AFRICA" 2008; West African Journal of Medicine (in press)
- ^ Greenwood BM, Wali SS. Control of meningococcal infection in the African meningitis belt by selective vaccination. Lancet 1980;1:729-32
- ^ Kirsch EA, Barton RP, Kitchen L et al. Pathophysiology, treatment and outcome of meningococcemia: a review and recent experience. Pediatr Infect Dis J. 1996; 15:967-79
- ^ Ross SC, Densen P. Complement deficiency states and infection: epidemiology, pathogenesis and consequences of neisserial and other infections in an immune deficiency. Medicine (Baltimore). 1984; 63:243-73
- ^ Orren A, Potter PC, Cooper RC et al. Deficiency of the sixth component of complement and susceptibility to Neisseria meningitidis infections: studies in 10 families and five isolated cases. Immunology. 1987; 62:249-53
- ^ Ross SC, Rosenthal PJ, Berberich HM et al. Killing of Neisseria meningitidis by human neutrophils: implications for normal and complement-deficient individuals. J Infect Dis. 1987; 155:1266-75
- ^ Ross SC, Berberich HM, Densen P. Natural serum bactericidal activity against Neisseria meningitidis isolates from disseminated infections in normal and complement-deficient hosts. J Infect Dis. 1985; 152:1332-5
- ^ Ala Aldeen DAA, Cartwright KAV. Neisseria meningitidis: vaccines and vaccine candidates. J Infect. 1996; 33:153-7
- ^ Mayon-White RT, Heath PT. Preventative strategies on meningococcal disease. Arch Dis Child. 1997; 76:178-81
- ^ Ross SC, Densen P. Complement deficiency states and infection: epidemiology, pathogenesis and consequences of neisserial and other infections in an immune deficiency. Medicine (Baltimore). 1984; 63:243-73
- ^ Andreoni J, Käyhty H, Densen P. Vaccination and the role of capsular polysaccharide antibody in prevention of recurrent meningococcal disease in late complement component-deficient individuals. J Infect Dis. 1993; 68:227-31
- ^ Cunliffe NA, Snowden N, Dunbar EW et al. Recurrent meningococcal septicemia and properdin deficiency. J Infect Dis. 1995; 31:67-8
- ^ Kirsch EA, Barton RP, Kitchen L et al. Pathophysiology, treatment and outcome of meningococcemia: a review and recent experience. Pediatr Infect Dis J. 1996; 15:967-79
- ^ Cunliffe NA, Snowden N, Dunbar EW et al. Recurrent meningococcal septicemia and properdin deficiency. J Infect Dis. 1995; 31:67-8
- ^ Kirsch EA, Barton RP, Kitchen L et al. Pathophysiology, treatment and outcome of meningococcemia: a review and recent experience. Pediatr Infect Dis J. 1996; 15:967-79
- ^ Cunliffe NA, Snowden N, Dunbar EW et al. Recurrent meningococcal septicemia and properdin deficiency. J Infect Dis. 1995; 31:67-8
- ^ Kirsch EA, Barton RP, Kitchen L et al. Pathophysiology, treatment and outcome of meningococcemia: a review and recent experience. Pediatr Infect Dis J. 1996; 15:967-79
- ^ Centers for Disease Control and Prevention. Prevention and control of serogroup C meningococcal disease and meningococcal disease and college students: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2000; 49(No. RR-7):1-20
- ^ Centers for Disease Control and Prevention. Recommendations of the Advisory Committee on Immunization Practices (ACIP): use of vaccines and immune globulins in persons with altered immunocompetence. MMWR Morb Mortal Wkly Rep. 1993; 42(RR-4):1-18
External links
Categories:- Bacterial diseases
- Bacterium-related cutaneous conditions
Wikimedia Foundation. 2010.