- Acute respiratory distress syndrome
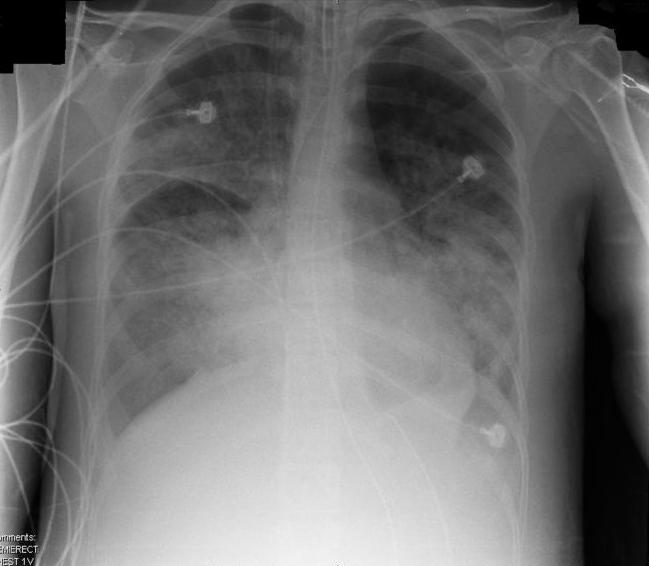
Caption =Chest x-ray of patient with ARDS
OMIM =
MedlinePlus = 000103
eMedicineSubj = med
eMedicineTopic = 70
DiseasesDB = 892
MeshID = D012128Acute respiratory distress syndrome (ARDS), also known as respiratory distress syndrome (RDS) or adult respiratory distress syndrome (in contrast with IRDS) is a serious reaction to various forms of injuries to the
lung .ARDS is a severe
lung disease caused by a variety of direct and indirect issues. It is characterized byinflammation of the lungparenchyma leading to impairedgas exchange with concomitant systemic release ofinflammatory mediator s causinginflammation , hypoxemia and frequently resulting inmultiple organ failure . This condition is often lethal, usually requiringmechanical ventilation and admission to anintensive care unit . A less severe form is calledacute lung injury (ALI).ARDS formerly most commonly signified "adult respiratory distress syndrome" to differentiate it from
infant respiratory distress syndrome in premature infants. However, as this type of pulmonary edema also occurs in children, "ARDS" has gradually shifted to mean "acute" rather than "adult". The differences with the typical infant syndrome remain.Definition
"Historical background"
Acute respiratory distress syndrome was first described in 1967 by Ashbaugh "et al".cite book | author = Irwin RS, Rippe JM | title = Irwin and Rippe's Intensive Care Medicine | edition = 5th ed. | publisher = Lippincott Williams & Wilkins | year = 2003 | id = ISBN 0-7817-3548-3 ] cite journal | author = Ashbaugh D, Bigelow D, Petty T, Levine B | title = Acute respiratory distress in adults | journal = Lancet | volume = 2 | issue = 7511 | pages = 319–23 | year = 1967 | pmid = 4143721 | doi = 10.1016/S0140-6736(67)90168-7] Initially there was no definition, resulting in controversy over incidence and mortality. In
1988 an expanded definition was proposed which quantified physiologic respiratory impairment.In 1994 a new definition was recommended by the American-European Consensus Conference Committee.cite journal | author = Bernard G, Artigas A, Brigham K, Carlet J, Falke K, Hudson L, Lamy M, Legall J, Morris A, Spragg R | title = The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination | journal = Am J Respir Crit Care Med | volume = 149 | issue = 3 Pt 1 | pages = 818–24 | year = 1994 | pmid = 7509706] It had two advantages: 1 it recognizes that severity of pulmonary injury varies, 2 it is simple to use.cite journal | author = Ware L, Matthay M | title = The acute respiratory distress syndrome | journal = N Engl J Med | volume = 342 | issue = 18 | pages = 1334–49 | year = 2000 | pmid = 10793167 | doi = 10.1056/NEJM200005043421806]
ARDS was defined as the ratio of arterial partial oxygen tension (PaO2) as fraction of inspired oxygen (FiO2) below 200 mmHg in the presence of bilateral
alveolar infiltrate s on the chest x-ray. These infiltrates may appear similar to those of left ventricular failure, but the cardiac silhouette appears normal in ARDS. Also, the pulmonary capillary wedge pressure is normal (less than 18 mmHg) in ARDS, but raised in left ventricular failure.A PaO2/FiO2 ratio less than 300 mmHg with bilateral infiltrates indicates
acute lung injury (ALI). Although formally considered different from ARDS, ALI is usually just a precursor to ARDS."Consensus after 1967 and 1994"
ARDS is characterized by:
* Acute onset
* Bilateral infiltrates on chest radiograph
*Pulmonary artery wedge pressure < 18 mmHg (obtained bypulmonary artery catheter ization), if this information is available; if unavailable, then lack of clinical evidence of left ventricular failure suffices
* if PaO2:FiO2 < 300 mmHgacute lung injury (ALI) is considered to be present
* if PaO2:FiO2 < 200 mmHg acute respiratory distress syndrome (ARDS) is considered to be presentPatient presentation and diagnosis
ARDS can occur within 24 to 48 hours of an injury or attack of acute illness. In such a case the patient usually presents with
shortness of breath ,tachypnea , and symptoms related to the underlying cause, i.e. shock. ARDS is classically associated with hypoxemia,petechiae in the axillae and neurologic abnomalities such as mental confusion.Long term illnesses can also trigger it, eg malaria. The ARDS may then occur sometime after the onset of a particularly acute case of the infection. See [http://www.medicine.mcgill.ca/tropmed/cantropmed/image34.htm xray of malarial ARDS] .
An
arterial blood gas analysis andchest X-ray allow formal diagnosis by inference using the aforementioned criteria. Although severe hypoxemia is generally included, the appropriate threshold defining abnormal PaO2 has never been systematically studied.Any cardiogenic cause of pulmonary edema should be excluded. This can be done by placing a
pulmonary artery catheter for measuring the pulmonary artery wedge pressure. However, this is not necessary and is now rarely done as abundant evidence has emerged demonstrating that the use of pulmonary artery catheters does not lead to improved patient outcomes in critical illness including ARDS.Plain Chest X-rays are sufficient to document bilateral alveolar infiltrates in the majority of cases. While CT scanning leads to more accurate images of the pulmonary parenchyma in ARDS, it has little utility in the clinical management of patients with ARDS, and remains largely a research tool.
Pathophysiology
ARDS is characterized by a diffuse inflammation of lung parenchyma. The triggering insult to the parenchyma usually results in an initial release of
cytokines and other inflammatory mediators, secreted by local epithelial and endothelial cells.Neutrophils and some T-lymphocytes quickly migrate into the inflamed lung parynchema and contribute in the amplification of the phenomenon.Typical histological presentation involves diffuse alveolar damage and
hyaline membrane formation in alveolar walls.Although the triggering mechanisms are not completely understood, recent research has examined the role of inflammation and mechanical stress.
Inflammation
Inflammation alone, as in sepsis, causes endothelial dysfunction, fluid extravasation from the capillaries and impaired drainage of fluid from the lungs. Dysfunction of type II pulmonary epithelial cells may also be present, with a concomitant reduction in
surfactant production. Elevated inspired oxygen concentration often becomes necessary at this stage, and they may facilitate a 'respiratory burst ' in immune cells.In a secondary phase, endothelial dysfunction causes cells and inflammatory exudate to enter the alveoli. This
pulmonary edema increases the thickness of the alveolo-capillary space, increasing the distance theoxygen must diffuse to reachblood . This impairs gas exchange leading to hypoxia, increases the work of breathing, eventually inducesfibrosis of the airspace.Moreover, edema and decreased surfactant production by type II pneumocytes may cause whole alveoli to collapse, or to completely flood. This "loss of aeration" contributes further to the
right-to-left shunt in ARDS. As the alveoli contain progressively less gas, more blood flows through them without being oxygenated resulting in massive intrapulmonary shunting.Collapsed alveoli (and small
bronchi ) do not allow gas exchange. It is not uncommon to see patients with a PaO2 of 60mmHg (8.0 kPa) despite mechanical ventilation with 100% inspired oxygen.The loss of aeration may follow different patterns according to the nature of the underlying disease, and other factors. In pneumonia-induced ARDS, for example, large, more commonly causes relatively compact areas of alveolar infiltrates. These are usually distributed to the lower lobes, in their posterior segments, and they roughly correspond to the initial infected area.
In sepsis or trauma-induced ARDS, infiltrates are usually more patchy and diffuse. The posterior and basal segments are always more affected, but the distribution is even less homogeneous.
Loss of aeration also causes important changes in lung mechanical properties. These alterations are fundamental in the process of inflammation amplification and progression to ARDS in mechanically ventilated patients.
Mechanical stress
Mechanical ventilation is an essential part of the treatment of ARDS. As loss of aeration (and the underlying disease) progress, thework of breathing (WOB) eventually grows to a level incompatible with life. Thus, mechanical ventilation is initiated to relieve respiratory muscles of their work, and to protect the usually obtunded patient'sairway s.However, mechanical ventilation may constitute a risk factor for the development, or the worsening, of ARDS.
Aside from the infectious complications arising from invasive ventilation with tracheal
intubation , positive-pressure ventilation directly alters lung mechanics during ARDS. The result is higher mortality, i.e. through baro-trauma, when these techniques are used.In 1998, Amato "et al" published a paper showing substantial improvement in the outcome of patients ventilated with lower
tidal volume s ("V"t) (6 mL·kg-1).cite journal | author = Amato M, Barbas C, Medeiros D, Magaldi R, Schettino G, Lorenzi-Filho G, Kairalla R, Deheinzelin D, Munoz C, Oliveira R, Takagaki T, Carvalho C | title = Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome | journal = N Engl J Med | volume = 338 | issue = 6 | pages = 347–54 | year = 1998 | pmid = 9449727 | doi = 10.1056/NEJM199802053380602] This result was confirmed in a 2000 study sponsored by the NIH.cite journal | author = MacIntyre N | title = Mechanical ventilation strategies for lung protection | journal = Semin Respir Crit Care Med | volume = 21 | issue = 3 | pages = 215–22 | year = 2000 | pmid = 16088734 | doi = 10.1055/s-2000-9850] Although both these studies were widely criticized for several reasons, and although the authors were not the first to experiment lower-volume ventilation, they shed new light on the relationship between mechanical ventilation and ARDS.One opinion is that the forces applied to the lung by the
ventilator may work as a lever to induce further damage to lung parenchyma. It appears thatshear stress at the interface between collapsed and aerated units may result in the breakdown of aerated units, which inflate asymmetrically due to the 'stickiness' of surrounding flooded alveoli. The fewer such interfaces around an alveolus, the lesser the stress.Indeed, even relatively low stress forces may induce
signal transduction systems at the cellular level, thus inducing the release of inflammatory mediators.This form of stress is thought to be applied by the
transpulmonary pressure (gradient ) ("P"l) generated by the ventilator or, better, its cyclical variations. The better outcome obtained in patients ventilated with lower "V"t may be interpreted as a beneficial effect of the lower "P"l. Transpulmonarypressure , is an indirect function of the "V"t setting on the ventilator, and only trial patients withplateau pressure s (a surrogate for the actual "P"l) were less than 32 cmH2O (3.1 kPa) had improved survival.The way "P"l is applied on alveolar surface determines the shear stress to which lung units are exposed. ARDS is characterized by an usually inhomogeneous reduction of the airspace, and thus by a tendency towards higher "P"l at the same "V"t, and towards "higher" stress on "less" diseased units.
The inhomogeneity of alveoli at different stages of disease is further increased by the gravitational gradient to which they are exposed, and the different
perfusion pressure s at which blood flows through them. Finally, abdominal pressure exerts an additional pressure on inferoposterior lung segments, favoring compression and collapse of those units.The different mechanical properties of alveoli in ARDS may be interpreted as having varying "time constants" (the product of alveolar compliance × resistance). A long time constant indicates an alveolus which opens slowly during tidal inflation, as a consequence of contrasting pressure around it, or altered water-air interface inside it (loss of surfactant, flooding).
Slow alveoli are said to be 'kept open' using positive end-expiratory pressure, a feature of modern ventilators which maintains a positive airway pressure throughout the whole respiratory cycle. A higher mean pressure cycle-wide slows the collapse of diseased units, but it has to be weighed against the corresponding elevation in "P"l/plateau pressure.
The prone position also reduces the inhomogeneity in alveolar time constants induced by gravity and edema.
Progression
If the underlying disease or injurious factor is not removed, the amount of inflammatory mediators released by the lungs in ARDS may result in a
systemic inflammatory response syndrome (or sepsis if there is lung infection). The evolution towards shock and/ormultiple organ failure follows paths analogous to the pathophysiology of sepsis.This adds up to the impaired oxygenation, the real mainstay of ARDS, and
respiratory acidosis , often caused by the ventilation techniques indicated in ARDS.The result is a critical illness in which the 'endothelial disease' of severe sepsis/
SIRS is worsened by the pulmonary dysfunction, which further impairs oxygen delivery.Treatment
General
Acute respiratory distress syndrome is usually treated with
mechanical ventilation in the Intensive Care Unit. Ventilation is usually delivered through oro-trachealintubation , or tracheostomy whenever prolonged ventilation (≥2 weeks) is deemed inevitable.The possibilities of
non-invasive ventilation are limited to the very early period of the disease or, better, to prevention in individuals at risk for the development of the disease (atypical pneumonia s,pulmonary contusion , major surgery patients).Treatment of the underlying cause is imperative, as it tends to maintain the ARDS picture.
Appropriate
antibiotic therapy must be administered as soon asmicrobiological culture results are available.Empirical therapy "may" be appropriate if local microbiological surveillance is efficient. More than 60% ARDS patients experience a (nosocomial ) pulmonary infection either before or after the onset of lung injury.The origin of
infection , when surgically treatable, must be operated on. Whensepsis is diagnosed, appropriate local protocols should be enacted.Commonly used supportive therapy includes particular techniques of mechanical ventilation and pharmacological agents whose effectiveness with respect to the outcome has not yet been proven. It is now debated whether mechanical ventilation is to be considered mere supportive therapy or actual treatment, since it may substantially affect survival.
Mechanical ventilation
The overall goal is to maintain acceptable gas exchange and to minimize adverse effects in its application. Three parameters are used: PEEP (positive end-expiratory pressure, to maintain maximal recruitment of alveolar units), mean airway pressure (to promote recruitment and predictor of hemodynamic effects) and plateau pressure (best predictor of alveolar overdistention). [cite journal | author =Malhotra A | title = Low-tidal-volume ventilation in the acute respiratory distress syndrome | journal = N Engl J Med | volume = 357 | issue = 11 | pages = 1113–20| year = 2007 | pmid = 17855672 | doi =10.1056/NEJMct074213]
Conventional therapy aimed at
tidal volume s ("V"t) of 12-15 ml/kg. Recent studies have shown that high tidal volumes can overstretch alveoli resulting involutrauma (secondary lung injury). The ARDS Clinical Network, or [http://www.ardsnet.org/index.php ARDSNet] , completed a landmark trial that showed improved mortality when ventilated with a tidal volume of 6 ml/kg compared to the traditional 12 ml/kg. Low tidal volumes ("V"t) may cause hypercapnia andatelectasis .Low tidal volume ventilation was the primary independent variable associated with reduced mortality in the NIH-sponsored ARDSnet trial of tidal volume in ARDS. Plateau pressure less than 30 cm H2O was a secondary goal, and subsequent analyses of the data from the ARDSnet trial (as well as other experimental data) demonstrate that there appears to be NO safe upper limit to plateau pressure; that is, regardless of plateau pressure, patients fare better with low tidal volumes (see Hager et al, American Journal of Respiratory and Critical Care Medicine, 2005).
APRV (Airway Pressure Release Ventilation) and ARDS / ALI
Although a particular ventilation mode has yet to be "proven in clinical trials"* more effective than others in treating patients with ARDS, ever increasing empirical evidence and clinical experience is showing that [http://www.aacn.org/pdfLibra.NSF/Files/ci120205/$file/ci120205.pdf APRV] is the primary mode of choice when ventilating a patient with ARDS or ALI (Acute Lung Injury).
Advantages to APRV ventilation include: decreased airway pressures, decreased minute ventilation, decreased dead-space ventilation, promotion of spontaneous breathing, almost 24 hour a day alveolar recruitment, decreased use of sedation, near elimination of neuromuscular blockade and an often positive effect on cardiac output (due to the negative inflection from the elevated baseline with each spontaneous breath).
A patient with ARDS on average spends 8 to 11 days on a mechanical ventilator; APRV may reduce this time significantly.Fact|date=May 2008
* *This would require a side by side study of APRV and the current ARDSNet protocol. There seems to be little political will, within the medical community, to address the need for this study, in spite of the successes seen with APRV.
Positive end-expiratory pressure
Positive end-expiratory pressure (PEEP) must be used in mechanically-ventilated patients in order to contrast the tendency to collapse of affected alveoli.Ideally, a 'perfect' PEEP would match the increased alveolar
surface tension , caused by surfactant deficiency and external pressure (edema), thus restoring a normal time constant in all affected units.However, because of the cited inherent inhomogeneity, surface tension varies, and so do PEEP requirements for the diseased units. Furthermore, high levels of PEEP may impair
venous blood return to the rightheart , although the actual impact of PEEP onhemodynamics is still debated.The 'best PEEP' used to be defined as 'some' cmH2O above the lower inflection point (LIP) in the sigmoidal pressure-volume relationship curve of the lung. Recent research has shown that the LIP-point pressure is no better than any pressure above it, as recruitment of collapsed alveoli, and more importantly the overdistension of aerated units, occur throughout the whole inflation. Despite the awkwardness of most procedures used to trace the pressure-volume curve, it is still used by some to define the "minimum" PEEP to be applied to their patients. Some of the newest ventilators have the ability to automatically plot a pressure-volume curve. The possibility of having an 'instantaneous' tracing trigger might produce renewed interest in this analysis.
PEEP may also be set empirically. Some authors suggest performing a 'recruiting maneuver' (i.e., a short time at a very high continuous positive airway pressure, such as 50 cmH2O (4.9 kPa), to recruit, or open, collapsed unit with a high distending pressure) and then to increase PEEP to a rather high level before restoring previous ventilation. The final PEEP level should be the one just before the drop in PaO2 (or peripheral blood oxygen saturation) during a step-down trial.
PEEP 'stacks up' to Pl during volume-controlled ventilation. At high levels, it may cause significant overdistension of (and injury to) compliant, aerated units, and higher plateau pressures at the same "V"t.
Intrinsic PEEP (iPEEP), or auto-PEEP, is not detected during normal ventilation. However, when ventilating at high frequencies, its contribution may be substantial, both in its positive and negative effects. There are 'underground', unproven claims that the Amato and NIH/ARDS Network studies got a positive result because of the high iPEEP levels reached by spontaneously breathing patients in low-volume assist-control ventilation. Whether or not that is true, it is a fact that iPEEP has been measured in very few formal studies on ventilation in ARDS patients, and its entity is largely unknown. Its measurement is recommended in the treatment of ARDS patients, especially when using high-frequency (oscillatory/jet) ventilation.
A compromise between the beneficial and adverse effects of PEEP is, as usual, inevitable.
Prone position
Distribution of lung infiltrates in acute respiratory distress syndrome is non-uniform. Repositioning into the prone position (face down) might improve oxygenation by relieving
atelectasis and improving perfusion. However, although the hypoxemia is overcome there seems to be no effect on overall survival.cite journal | author = Gattinoni L, Tognoni G, Pesenti A, Taccone P, Mascheroni D, Labarta V, Malacrida R, Di Giulio P, Fumagalli R, Pelosi P, Brazzi L, Latini R | title = Effect of prone positioning on the survival of patients with acute respiratory failure | journal = N Engl J Med | volume = 345 | issue = 8 | pages = 568–73 | year = 2001 | pmid = 11529210 | doi = 10.1056/NEJMoa010043]Fluid management
Several studies have shown that pulmonary function and outcome are better in patients that lost weight or
wedge pressure was lowered bydiuresis or fluid restriction.Corticosteroids
A Meduri et al study has found significant improvement in ARDS using modest doses of corticosteroids. This is probably because of a suppression of ongoing inflammation during the fibroproliferative phase of ARDS. The initial regimen consists of
methylprednisolone 2 mg/kg daily. After 3-5 days a response must be apparent. In 1-2 weeks the dose can be tapered to methylprednisolone 0.5-1.0 mg daily. Patients with ARDS do not benefit from high-dose corticosteroids.cite journal | author = Meduri G, Tolley E, Chrousos G, Stentz F | title = Prolonged methylprednisolone treatment suppresses systemic inflammation in patients with unresolving acute respiratory distress syndrome: evidence for inadequate endogenous glucocorticoid secretion and inflammation-induced immune cell resistance to glucocorticoids | journal = Am J Respir Crit Care Med | volume = 165 | issue = 7 | pages = 983–91 | year = 2002 | pmid = 11934726]The recent NIH-sponsored ARDSnet LAZARUS study of corticosteroids for ARDS demonstrated that they are not efficacious in ARDS.
Nitric oxide
Inhaled
nitric oxide (NO) potentially acts as selective pulmonary vasodilator. Rapid binding tohemoglobin prevents systemic effects. It should increase perfusion of better ventilated areas. There are no large studies demonstrating positive results. Therefore its use must be considered individually.Almitrine bismesylate stimulates chemoreceptors in carotic and aortic bodies. It has been used to potentiate the effect of NO, presumably by potentiating hypoxia-induced pulmonary vasoconstriction. In case of ARDS it is not known whether this combination is useful.Surfactant therapy
To date no prospective controlled clinical trial has shown a significant mortality benefit of exogenous surfactant in ARDS.
Complications
Since ARDS is an extremely serious condition which requires invasive forms of therapy it is not without risk. Complications to be considered are:
*Pulmonary:barotrauma (volutrauma),pulmonary embolism (PE), pulmonary fibrosis,ventilator-associated pneumonia (VAP).
*Gastrointestinal: hemorrhage (ulcer), dysmotility, pneumoperitoneum, bacterial translocation.
*Cardiac: arrhythmias, myocardial dysfunction.
*Renal:acute renal failure (ARF), positive fluid balance.
*Mechanical: vascular injury, pneumothorax (by placing pulmonary artery catheter), tracheal injury/stenosis (result of intubation and/or irritation by endotracheal tube.
*Nutritional: malnutrition (catabolic state), electrolyte deficiency.Epidemiology
The annual incidence of ARDS is 1.5–13.5 people per 100,000 in the general population.fact|date=June 2008 Its incidence in the
intensive care unit (ICU), mechanically ventilated population is much higher. Brun-Buisson "et al." (2004) reported a prevalence of acute lung injury (ALI) (see below) of 16.1% percent in ventilated patients admitted for more than 4 hours. More than half these patients may develop ARDS.Mechanical ventilation ,sepsis ,pneumonia , shock, aspiration, trauma (especiallypulmonary contusion ), major surgery, massive transfusions,smoke inhalation , drug reaction oroverdose ,fat emboli and reperfusion pulmonary edema afterlung transplantation or pulmonary embolectomy may all trigger ARDS. Pneumonia and sepsis are the most common triggers, and pneumonia is present in up to 60% of patients. Pneumonia and sepsis may be either causes or complications of ARDS.Elevated
abdominal pressure of any cause is also probably a risk factor for the development of ARDS, particularly during mechanical ventilation.The
mortality rate varies from 30% to 60%.fact|date=June 2008 Usually,randomized controlled trials in the literature show lower death rates, both in control and treatment patients. This is thought to be due to stricter enrollment criteria. Observational studies generally report 50%–60% mortality.fact|date=June 2008References
Further reading
*
*
*External links
* [http://www.aafp.org/afp/20020501/1823.html Acute Respiratory Distress Syndrome - May 1, 2002 - American Family Physician]
* [http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=10193545&query_hl=62&itool=pubmed_docsum Antioxidant status in patients with acute respiratory distress syndrome.]
* [http://www.ardsnet.org/ ARDSNet] — the NIH / NHLBI ARDS Network
* [http://www.ards.org/ ARDS Support Center] — information and support for patients with ARDS and their loved ones
* [http://www.ardsusa.org/ ARDS Foundation] — a charitable organization offers support to families/victims of Acute Respiratory Distress Syndrome"
Wikimedia Foundation. 2010.