- Metalloprotein
-
Metalloprotein is a generic term for a protein that contains a metal ion cofactor.[1] Metalloproteins have many different functions in cells, such as enzymes, transport and storage proteins, and signal transduction proteins. Indeed, about one quarter to one third of all proteins require metals to carry out their functions.[2] The metal ion is usually coordinated by nitrogen, oxygen or sulfur atoms belonging to amino acids in the polypeptide chain and/or a macrocyclic ligand incorporated into the protein. The presence of the metal ion allows metalloenzymes to perform functions such as redox reactions that cannot easily be performed by the limited set of functional groups found in amino acids.[3]
Contents
Storage and transport metalloproteins
Oxygen carriers
Hemoglobin, which is the principal oxygen carrier in humans has four sub-units in which the iron(II) ion is coordinated by the planar, macrocyclic ligand protoporphyrin IX (PIX) and the imidazole nitrogen atom of a histidine residue. The sixth coordination site contains a water molecule or a dioxygen molecule. myoglobin has only one such unit. The active site is located in an hydrophobic pocket. This is important as, without it, the iron(II) would be irreversibly oxidised to iron(III). The equilibrium constant for the formation of HbO2 is such that oxygen is taken up or released depending on the partial pressure of oxygen in the lungs or in muscle. In hemoglobin the four sub-units show a cooperativity effect which allows for easy oxygen transfer from hemoglobin to myoglobin.[4]
In both hemoglobin and myoglobin it is sometimes incorrectly stated that the oxygenated species contains iron(III). It is now known that the diamagnetic nature of these species is due to the fact that the iron(II) is in the low-spin state. In oxyhemoglobin the iron atom is located in the plane of the porphyrin ring, but in the paramagnetic deoxyhemoglobin the iron atom lies above the plane of the ring. [4] The change in spin state is a cooperative effect of higher crystal field splitting and smaller ionic radius of Fe2+ in the oxy- moiety.
Hemerythrin is another iron-containing oxygen carrier. The oxygen binding site is a binuclear iron center. The iron atoms are coordinated to the protein through the carboxylate side chains of a glutamate and aspartate and five histidine residues. The uptake of O2 by hemerythrin is accompanied by two-electron oxidation of the reduced binuclear center to produce bound peroxide (OOH-). The mechanism of oxygen uptake and release have been worked out in detail.[5][6]
Hemocyanins carry oxygen in the blood of most molluscs, and some arthropods such as the horseshoe crab. They are second only to hemoglobin in biological popularity of use in oxygen transport. On oxygenation the two copper(I) atoms at the active site are oxidised to copper(II) and the dioxygen molecules is reduced to peroxide, O22-.[7][8]
Cytochromes
Oxidation and reduction reactions are not common in organic chemistry as few organic molecules can act as oxidizing or reducing agents. Iron(II), on the other hand, can easily be oxidized to iron(III). This functionality is used in cytochromes which function as electron-transfer vectors. The iron atom in most cytochromes is contained in a heme group. The differences between those cytochromes lies in the different side-chains. For instance Cytochrome a has a heme a prosthetic group and cytochrome b has a heme b prosthetic group. These differences result in different Fe2+/Fe3+ redox potentials such that various cytochromes are involved in the mitochondrial electron transport chain.[9]
Cytochrome P450 enzymes perform the function of inserting an oxygen atom into a C—H bond, an oxidation reaction.[10][11]
Rubredoxin
Rubredoxin is an electron-carrier found in sulfur-metabolizing bacteria and archaea. The active site contains an iron ion which is coordinated by the sulphur atoms of four cysteine residues forming an almost regular tetrahedron. Rubredoxins perform one-electron transfer processes. The oxidation state of the iron atom changes between the +2 and +3 states. In both oxidation states the metal is high spin, which helps to minimize structural changes.
Plastocyanin
Plastocyan is one of the family of blue copper proteins which are involved in electron transfer reactions. The copper binding site is described as a ‘distorted trigonal pyramidal’.[12] The trigonal plane of the pyramidal base is composed of two nitrogen atoms (N1 and N2) from separate histidines and a sulfur (S1) from a cysteine. Sulfur (S2) from an axial methionine forms the apex. The ‘distortion’ occurs in the bond lengths between the copper and sulfur ligands. The Cu-S1 contact is shorter (207 picometers) than Cu-S2 (282 pm). The elongated Cu-S2 bonding destabilises the CuII form and increases the redox potential of the protein. The blue colour (597 nm peak absorption) is due to the Cu-S1 bond where Spπ to Cudx2-y2 charge transfer occurs.[13]
In the reduced form of plastocyanin, His-87 will become protonated with a pKa of 4.4. Protonation prevents it acting as a ligand and the copper site geometry becomes trigonal planar.
Iron storage and transfer
Iron is stored as iron(III) in ferritin. The exact nature of the binding site has not yet been determined. The iron appears to be present as an hydrolysis product such as FeO(OH). Iron is transported by transferrin whose binding site consists of two tyrosines, one aspartic acid and one histidine.[14]
The human body has no mechanism for iron excretion. This can lead to iron-overload problems in patients treated with blood transfusions, as, for instance, with β-thallasemia.
Ceruloplasmin
Ceruloplasmin is the major copper-carrying protein in the blood. Ceruloplasmin exhibits oxidase activity, which is associated with possible oxidation of Fe2+ (ferrous iron) into Fe3+ (ferric iron), therefore assisting in its transport in the plasma in association with transferrin, which can only carry iron in the ferric state.
Metalloenzymes
Metalloenzymes all have one feature in common, namely, that the metal ion is bound to the protein with one labile coordination site. As with all enzymes, the shape of the active site is crucial. The metal ion is usually located in a pocket whose shape fits the substrate. The metal ion catalyzes reactions which are difficult to achieve in organic chemistry.
Carbonic anhydrase
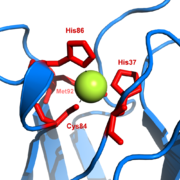
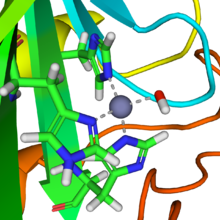 Active site of carbonic anhydrase. The three coordinating histidine residues are shown in green, hydroxide in red and white, and the zinc in gray.
Active site of carbonic anhydrase. The three coordinating histidine residues are shown in green, hydroxide in red and white, and the zinc in gray.
This reaction is very slow in the absence of a catalyst, but quite fast in the presence of the hydroxide ion
A reaction similar to this is almost instantaneaous with carbonic anhydrase. The structure of the active site in carbonic anhydrases is well-known from a number of crystal structures. It consists of a zinc ion coordinated by three imidazole nitrogen atoms from three histidine units. The fourth coordination site is occupied by a water molecule. The coordination sphere of the zinc ion is approximately tetrahedral. The positively charged zinc ion polarizes the coordinated water molecule and nucleophilic attack by the negatively charged hydoxide portion on carbon dioxide (carbonic anhydride) proceeds rapidly. The catalytic cycle produces the bicarbonate ion and the hydrogen ion[1] as the equilibrium
favours dissociation of carbonic acid at biological pH values.[15]
Vitamin B12-dependent enzymes
Vitamin B12 catalyzes the transfer of methyl (-CH3) groups between two molecules, which involves the breaking of C-C bonds, a process that is energetically expensive in organic reactions. The metal ion lowers the activation energy for the process by forming a transient Co-CH3 bond.[16] The structure of the coenzyme was famously determined by Dorothy Hodgkin and co-workers, for which she received a Nobel prize.[17] It constists of a cobalt(II) ion coordinated by four nitrogen atoms of a corrin rings and a fifth Nitrogen atom from an imidazole group. In the resting state there is a Co—C σ bond with the 5' carbon atom of adenosine.[18] This is a naturally occurring organometallic compound, which explains its function in trans-methylation reactions, such as the reaction carried out by methionine synthase.
Nitrogenase (nitrogen fixation)
The fixation of atmospheric nitrogen is a very energy-intensive process, as it involves breaking the very stable triple bond between the nitrogen atoms. The enzyme nitrogenase is one of the few enzymes that can catalyze the process. The enzyme occurs in certain bacteria. There are three components to its action: a molybdenum atom at the active site, Iron-sulfur clusters which are involved in transporting the electrons needed to reduce the nitrogen and an abundant energy source. The energy is provided by a symbiotic relationship between the bacteria and a host plant, often a legume. The relationship is symbiotic because the plant supplies the energy by photosynthesis and benefits by obtaining the fixed nitrogen. The reaction may be written symbolically as
where Pi stands for inorganic phosphate. The precise structure of the active site has been difficult to determine. It appears to contain a MoFe7S8 cluster which is able to bind the dinitrogen molecule and, presumably, enable the reduction process to begin.[19] The electrons are transported by the associated "P" cluster, which contains two cubical Fe4S4 clusters joined by sulphur bridges.[20]
Superoxide dismutase
The superoxide ion, O2- is generated in biological systems by reduction of molecular oxygen. It has an unpaired electron, so it behaves as a free radical. It is a powerful oxidising agent. These properties render the superoxide ion very toxic and are deployed to advantage by phagocytes to kill invading micro organisms. Otherwise, the superoxide ion must be destroyed before it does unwanted damage in a cell. The superoxide dismutase enzymes perform this function very efficiently.[21]
The formal oxidation state of the oxygen atoms is ½. In solutions at neutral pH, the superoxide ion disproportionates to molecular oxygen and hydrogen peroxide.
- 2 O2− + 2 H+ → O2 + H2O2
In biology this type of reaction is call a dismutation reaction. It involves both oxidation and reduction of superoxide ions. The superoxide dismutase group of enzymes, abbreviated as SOD, increase the rate of reaction to near the diffusion limited rate.[22] The key to the action of these enzymes is a metal ion with variable oxidation state which can act as either an oxidizing agent or as a reducing agent.
- Oxidation: M(n+1)+ + O2− → Mn+ + O2
- Reduction: Mn+ + O2− + 2H+ → M(n+1)+ + H2O2.
In human SOD the active metal is copper, as Cu2+ or Cu+, coordinated tetrahedrally by four histidine residues. This enzyme also contains zinc ions. Other isozymes may contain iron, manganese or nickel. Ni-SOD is particularly interesting as it involves nickel(III), an unusual oxidation state for this element. The active site Ni geometry cycles from square planar Ni(II), with thiolate (Cys2 and Cys6) and backbone nitrogen (His1 and Cys2) ligands, to square pyramidal Ni(III) with an added axial His1 side chain ligand. [23]
Chlorophyll-containing proteins
Chlorophyll plays a crucial role in photosynthesis. It contains a magnesium enclosed in a chlorin ring. However, the magnesium ion is not directly involved in the photosynthetic function and can be replaced by other divalent ions with little loss of activity. Rather, the photon is absorbed by the chlorin ring, whose electronic structure is well-adapted for its purpose.
Initially, the absorption of a photon causes an electron to be excited into a singlet state of the Q band. The excited state undergoes an intersystem crossing from the singlet state to a triplet state in which there are two electrons with parallel spin. This species is, effectively, a free radical, and is very reactive and allows an electron to be transferred to acceptors which are adjacent to the chlorophyll in the chloroplast. In the process chlorophyll is oxidized. Later in the phosynthetic cycle, chlorophyll is re-reduced. This reduction ultimately draws electrons from water, yielding molecular oxygen as a final oxidation product.
Signal-transduction metalloproteins
Calmodulin
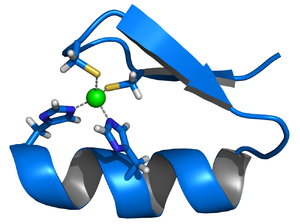
Calmodulin is an example of a signal-transduction protein. It is a small protein which contains four EF-hand motifs, each of which can bind a Ca2+ ion.
In an EF-hand loop the calcium ion is coordinated in a pentagonal bipyramidal configuration. Six Glutamic acid and Aspartic acid residues involved in the binding are in positions 1, 3, 5, 7, 9 of the polypeptide chain. At position 12 there is a glutamate or aspartate ligand which behaves as a (bidentate ligand), providing two oxygen atoms. The ninth residue in the loop is necessarily glycine due to the conformational requirements of the backbone. The coordination sphere of the calcium ion contains only carboxylate oxygen atoms and no nitrogen atoms. This is consistent with the hard nature of the calcium ion.
The protein has two approximately symmetrical domains, separated by a flexible "hinge" region. Binding of calcium causes a conformational change to occur in the protein. Calmodulin participates in an intracellular signalling system by acting as a diffusible second messenger to the initial stimuli.[24][25]
Transcription factors
Many transcription factors contain a structure known as a zinc finger, this is a structural module where a region of protein folds around a zinc ion. The zinc does not directly contact the DNA that these proteins bind to, instead the cofactor is essential for the stability of the tightly-folded protein chain.[26] In these proteins the zinc ion is usually coordinated by pairs of cysteine and histidine side chains.
Other metalloenzymes
Ion Examples of enzymes containing this ion Magnesium Glucose 6-phosphatase
Hexokinase
DNA polymeraseVanadium vanabins Manganese Arginase Iron Catalase
Hydrogenase
IRE-BP
AconitaseNickel[27] Urease
HydrogenaseCopper Cytochrome oxidase
LaccaseZinc Alcohol dehydrogenase
Carboxypeptidase
Aminopeptidase
Beta amyloidMolybdenum Nitrate reductase Selenium Glutathione peroxidase various Metallothionein
PhosphataseSee also
- Bioinorganic chemistry
- Coenzyme
- Cofactor
- Hemoproteins
- Metal Ions in Life Sciences
- Siderophore
- Prosthetic group
- QPNC-PAGE
References
- ^ a b Shriver, D.F.; Atkins, P.W. (1999). "Chapter 19, Bioinorganic chemistry". Inorganic chemistry (3rd. ed.). Oxford University Press. ISBN 019 850330x.
- ^ Waldron KJ, Robinson NJ (January 2009). "How do bacterial cells ensure that metalloproteins get the correct metal?". Nat. Rev. Microbiol. 7 (1): 25–35. doi:10.1038/nrmicro2057. PMID 19079350.
- ^ Messerschmidt, A; Huber, R.;Wieghardt,K.;Poulos, T. (2001). Handbook of Metalloproteins. Wiley. ISBN 0-471-62743-7.
- ^ a b Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Oxford: Butterworth-Heinemann. ISBN 0080379419. Fig.25.7, p 1100 illustrates the structure of deoxyhemoglobin
- ^ Stenkamp, R.E. (1994). "Dioxygen and hemerythrin". Chem. Rev. 94: 715–726. doi:10.1021/cr00027a008.
- ^ Wirstam M., Lippard, S.J., Friesner R.A. (2003). "Reversioble Dioxygen Binding to Hemerythrin". J. Am. Chem. Soc. 125 (13): 3980–3987. doi:10.1021/ja017692r. PMID 12656634.
- ^ K. D. Karlin, R. W. Cruse, Y. Gultneh, A. Farooq, J. C. Hayes and J. Zubieta (1987). "Dioxygen-copper reactivity. Reversible binding of O2 and CO to a phenoxo-bridged dicopper(I) complex". J. Am. Chem. Soc. 109 (9): 2668–2679. doi:10.1021/ja00243a019.
- ^ N. Kitajima, K. Fujisawa, C. Fujimoto, Y. Morooka, S. Hashimoto, T. Kitagawa, K. Toriumi, K. Tatsumi and A. Nakamura (1992). "A new model for dioxygen binding in hemocyanin. Synthesis, characterization, and molecular structure of the μ-η2:η2 peroxo dinuclear copper(II) complexes, [Cu(HB(3,5-R2pz)3)]2(O2) (R = isopropyl and Ph)". J. Am. Chem. Soc. 114 (4): 1277–1291. doi:10.1021/ja00030a025.
- ^ Moore, G.R.; Pettigrew, G.W. (1990). Cytochrome c:structural and physicochemical aspects. Berlin: Springer.
- ^ Astrid Sigel, Helmut Sigel and Roland K.O. Sigel, ed (2007). The Ubiquitous Roles of Cytochrome450 Proteins. Metal Ions in Life Sciences. 3. Wiley. ISBN 978-0-470-01672-5.
- ^ Ortiz de Montellano, P.R. (2005). Cytochrome P450 Structure, Mechanism, and Biochemistry (3rd. ed.). Springer. ISBN 978-0-306-48324-0.
- ^ Colman, P. M.; Freeman, H. C.; Guss, J. M.; Murata, M.; Norris, V. A.; Ramshaw, J. A. M.; Venkatappa, M. P. (1978). "X-Ray Crystal-Structure Analysis of Plastocyanin at 2.7 A Resolution". Nature 272 (5651): 319–324. doi:10.1038/272319a0.
- ^ Solomon, E.I.; Gewirth, A.A.; Cohen,S.L. (1986). "Spectroscopic Studies of Active Sites. Blue Copper and Electronic Structural Analogs". ACS Symposium Series 307: 236–266. doi:10.1021/bk-1986-0307.ch016.
- ^ Anderson BF, Baker HM, Dodson EJ, et al. (April 1987). "Structure of human lactoferrin at 3.2-A resolution". Proc. Natl. Acad. Sci. U.S.A. 84 (7): 1769–73. doi:10.1073/pnas.84.7.1769. PMC 304522. PMID 3470756. http://www.pnas.org/cgi/pmidlookup?view=long&pmid=3470756.
- ^ Lindskog S (1997). "Structure and mechanism of carbonic anhydrase". Pharmacol. Ther. 74 (1): 1–20. doi:10.1016/S0163-7258(96)00198-2. PMID 9336012.
- ^ Astrid Sigel, Helmut Sigel and Roland K.O. Sigel, ed (2008). Metal-carbon bonds in enzymes and cofactors. Metal Ions in Life Sciences. 6. Wiley. ISBN 978-1-84755-915-9.
- ^ "The Nobel Prize in Chemistry 1964". Nobelprize.org. http://nobelprize.org/nobel_prizes/chemistry/laureates/1964/index.html. Retrieved 2008-10-06.
- ^ Hodgkin, D.C. (1965). "The Structure of the Corrin Nucleus from X-ray Analysis". Proc. Roy. Soc. A 288: 294–305. doi:10.1098/rspa.1965.0219.
- ^ Orme-Johnson, W.H. (1993). Steifel, E.I; Coucouvannis, D.; Newton, D.C.. ed. Molybdenum enzymes, cofactors and model systems. Advances in chemystry, Symposium series no. 535. Washington, DC: American Chemical Society. pp. 257.
- ^ Chan, M.K.; Kim, J.; Rees, D.C. (1993). "The nitrogenase FeMo-cofactor and P-cluster pair: 2.2 A resolution structures". Science 260 (5109): 792. doi:10.1126/science.8484118. PMID 8484118.
- ^ Packer, L. (editor) (2002). Superoxide Dismutase: 349 (Methods in Enzymology). Academic Press. ISBN 0121822524.
- ^ Heinrich, Peter; Georg Löffler; Petro E. Petrides (2006). Biochemie und Pathobiochemie (Springer-Lehrbuch) (German Edition). Berlin: Springer. pp. 123. ISBN 3-540-32680-4.
- ^ Barondeau, D.P.; Kassmann C.J.; Bruns C.K.; Tainer J.A.; Getzoff E.D. (2004). "Nickel superoxide dismutase structure and mechanism". Biochemistry 43 (25): 8038–8047. doi:10.1021/bi0496081. PMID 15209499.
- ^ Stevens FC (1983). "Calmodulin: an introduction". Can. J. Biochem. Cell Biol. 61 (8): 906–10. doi:10.1139/o83-115. PMID 6313166.
- ^ Chin D, Means AR (2000). "Calmodulin: a prototypical calcium sensor". Trends Cell Biol. 10 (8): 322–8. doi:10.1016/S0962-8924(00)01800-6. PMID 10884684.
- ^ Berg JM (1990). "Zinc finger domains: hypotheses and current knowledge". Annu Rev Biophys Biophys Chem 19: 405–21. doi:10.1146/annurev.bb.19.060190.002201. PMID 2114117.
- ^ Astrid Sigel, Helmut Sigel and Roland K.O. Sigel, ed (2008). Nickel and Its Surprising Impact in Nature. Metal Ions in Life Sciences. 2. Wiley. ISBN 978-0-470-01671-8.
External links
Enzyme cofactors Active forms TPP / ThDP (B1) · FMN, FAD (B2) · NAD+, NADH, NADP+, NADPH (B3) · Coenzyme A (B5) · PLP / P5P (B6) · Biotin (B7) · THFA / H4FA, DHFA / H2FA, MTHF (B9) · AdoCbl, MeCbl (B12) · Ascorbic Acid (C) · Phylloquinone (K1), Menaquinone (K2) · Coenzyme F420ATP · CTP · SAMe · PAPS · GSH · Coenzyme B · Cofactor F430 · Coenzyme M · Coenzyme Q · Heme / Haem (A, B, C, O) · Lipoic Acid · Methanofuran · Molybdopterin/Molybdenum cofactor · PQQ · THB / BH4 · THMPT / H4MPTBase forms M: NUT
cof, enz, met
noco, nuvi, sysi/epon, met
drug(A8/11/12)
Categories:- Metalloproteins
Wikimedia Foundation. 2010.