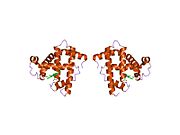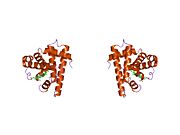- Myoglobin
-
Myoglobin 
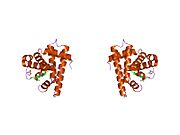
Model of helical domains in myoglobin.[1]Available structures PDB 1m6c, 1m6m, 1mdn, 1mnh, 1mni, 1mnj, 1mnk, 1mno, 1mwc, 1mwd, 1myg, 1myh, 1myi, 1myj, 1pmb, 1yca, 1ycb, 2mm1 Identifiers Symbols MB; MGC13548; PVALB External IDs OMIM: 160000 MGI: 96922 HomoloGene: 3916 GeneCards: MB Gene Gene Ontology Molecular function • oxygen transporter activity
• iron ion binding
• oxygen binding
• heme binding
• metal ion bindingBiological process • response to hypoxia
• transport
• oxygen transport
• enucleate erythrocyte differentiationSources: Amigo / QuickGO RNA expression pattern 
More reference expression data Orthologs Species Human Mouse Entrez 4151 17189 Ensembl ENSG00000198125 ENSMUSG00000018893 UniProt P02144 Q3UVB1 RefSeq (mRNA) NM_005368 NM_013593 RefSeq (protein) NP_005359 NP_038621 Location (UCSC) Chr 22:
34.33 – 34.35 MbChr 15:
76.84 – 76.88 MbPubMed search [1] [2] Myoglobin is an iron- and oxygen-binding protein found in the muscle tissue of vertebrates in general and in almost all mammals. It is related to hemoglobin, which is the iron- and oxygen-binding protein in blood, specifically in the red blood cells. The only time myoglobin is found in the bloodstream is when it is released following muscle injury. It is an abnormal finding, and can be diagnostically relevant when found in blood. [2]
Myoglobin (abbreviated Mb) is a single-chain globular protein of 153[3] or 154[4] amino acids, containing a heme (iron-containing porphyrin) prosthetic group in the center around which the remaining apoprotein folds. It has eight alpha helices and a hydrophobic core. It has a molecular weight of 17,699 daltons (with heme), and is the primary oxygen-carrying pigment of muscle tissues.[4] Unlike the blood-borne hemoglobin, to which it is structurally related,[5] this protein does not exhibit cooperative binding of oxygen, since positive cooperativity is a property of multimeric/oligomeric proteins only. Instead, the binding of oxygen by myoglobin is unaffected by the oxygen pressure in the surrounding tissue. Myoglobin is often cited as having an "instant binding tenacity" to oxygen given its hyperbolic oxygen dissociation curve. High concentrations of myoglobin in muscle cells allow organisms to hold their breaths longer. Diving mammals such as whales and seals have muscles with particularly high myoglobin abundance.[2]
Myoglobin was the first protein to have its three-dimensional structure revealed.[6] In 1958, John Kendrew and associates successfully determined the structure of myoglobin by high-resolution X-ray crystallography.[7] For this discovery, John Kendrew shared the 1962 Nobel Prize in chemistry with Max Perutz.[8] Despite being one of the most studied proteins in biology, its true physiological function is not yet conclusively established: mice genetically engineered to lack myoglobin are viable, but showed a 30% reduction in cardiac systolic output. They adapted to this deficiency through hypoxic genetic mechanisms and increased vasodilation.[9] In humans myoglobin is encoded by the MB gene.[10]
Contents
Meat color
Myoglobin forms pigments responsible for making meat red. The color that meat takes is partly determined by the oxidation states of the iron atom in myoglobin and the oxygen species attached to it. When meat is in its raw state, the iron atom is in the +2 oxidation state, and is bound to a dioxygen molecule (O2). Meat cooked well done is brown because the iron atom is now in the +3 oxidation state, having lost an electron, and is now coordinated by a water molecule. Under some conditions, meat can also remain pink all through cooking, despite being heated to high temperatures. If meat has been exposed to nitrites, it will remain pink because the iron atom is bound to NO, nitric oxide (true of, e.g., corned beef or cured hams). Grilled meats can also take on a pink "smoke ring" that comes from the iron binding to a molecule of carbon monoxide to give metmyoglobin.[11] Raw meat packed in a carbon monoxide atmosphere also shows this same pink "smoke ring" due to the same coordination chemistry. Notably, the surface of this raw meat also displays the pink color, which is usually associated in consumers' minds with fresh meat. This artificially-induced pink color can persist in the meat for a very long time, reportedly up to one year.[12] Hormel and Cargill are both reported to use this meat-packing process, and meat treated this way has been in the consumer market since 2003.[13] Myoglobin is found in Type I muscle, Type II A and Type II B, but most texts consider myoglobin not to be found in smooth muscle.
Role in disease
Myoglobin is released from damaged muscle tissue (rhabdomyolysis), which has very high concentrations of myoglobin. The released myoglobin is filtered by the kidneys but is toxic to the renal tubular epithelium and so may cause acute renal failure.[14] It is not the myoglobin itself that is toxic (it is a protoxin) but the ferrihemate portion that is dissociated from myoglobin in acidic environments (e.g., acidic urine, lysozymes).
Myoglobin is a sensitive marker for muscle injury, making it a potential marker for heart attack in patients with chest pain.[15] However, elevated myoglobin has low specificity for acute myocardial infarction (AMI) and thus CK-MB, cTnT, ECG, and clinical signs should be taken into account to make the diagnosis.
Structure and bonding
Myoglobin contains a porphyrin ring with an iron center. There is a proximal histidine group attached directly to the iron center, and a distal histidine group on the opposite face, not bonded to the iron.
Many functional models of myoglobin have been studied. One of the most important is that of picket fence porphyrin by James Collman. This model was used to show the importance of the distal prosthetic group. It serves three functions:
- To form hydrogen bonds with the dioxygen moiety, increasing the O2 binding constant
- To prevent the binding of carbon monoxide, whether from within or without the body. Carbon monoxide binds to iron in an end-on fashion, and is hindered by the presence of the distal histidine, which forces it into a bent conformation. CO binds to heme 23,000 times better than O2, but only 200 times better in hemoglobin and myoglobin. Oxygen binds in a bent fashion, which can fit with the distal histidine.[16]
- To prevent irreversible dimerization of the oxymyoglobin with another deoxymyoglobin species
See also
References
- ^ PDB 1MBO; Takano T (March 1977). "Structure of myoglobin refined at 2.0 Å resolution. II. Structure of deoxymyoglobin from sperm whale". J. Mol. Biol. 110 (3): 569–84. doi:10.1016/S0022-2836(77)80112-5. PMID 845960.
- ^ a b Nelson, D. L.; Cox, M. M. (2000). Lehninger Principles of Biochemistry (3rd ed.). New York: Worth Publishers. p. 206. ISBN 071676203X.
- ^ Hendgen-Cotta, U.; Kelm, M.; Rassaf, T. (2009). "A highlight of myoglobin diversity: the nitrite reductase activity during myocardial ischemia-reperfusion". Nitric oxide : biology and chemistry / official journal of the Nitric Oxide Society 22 (2): 75–82. doi:10.1016/j.niox.2009.10.003. PMID 19836457.
- ^ a b George A. Ordway and Daniel J. Garry (2004). "Myoglobin: an essential hemoprotein in striated muscle". Journal of Experimental Biology 207 (Pt 20): 3441–6. doi:10.1242/jeb.01172. PMID 15339940.
- ^ Harvey Lodish, Arnold Berk, Lawrence S. Zipursky, Paul Matsudaira, David Baltimore and James Darnell (2000). "Evolutionary tree showing the globin protein family members myoglobin and hemoglobin". Molecular Cell Biology (4th ed.). W. H. Freeman. ISBN 0-7167-3136-3. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Search&db=books&doptcmdl=GenBookHL&term=myoglobin+AND+mcb%5Bbook%5D+AND+105134%5Buid%5D&rid=mcb.figgrp.540.
- ^ (U.S.) National Science Foundation: Protein Data Bank Chronology (Jan. 21, 2004). Retrieved 3.17.2010
- ^ JC Kendrew, G Bodo, HM Dintzis, RG Parrish, H Wyckoff, and DC Phillips (1958). "A Three-Dimensional Model of the Myoglobin Molecule Obtained by X-Ray Analysis". Nature 181 (4610): 662–6. Bibcode 1958Natur.181..662K. doi:10.1038/181662a0. PMID 13517261.
- ^ The Nobel Prize in Chemistry 1962
- ^ Mammen PP, Kanatous SB, Yuhanna IS, Shaul PW, Garry MG, Balaban RS, Garry DJ (2003). "Hypoxia-induced left ventricular dysfunction in myoglobin-deficient mice". American Journal of Physiology. Heart and Circulatory Physiology 285 (5): H2132–41. doi:10.1152/ajpheart.00147.2003. PMID 12881221.
- ^ Akaboshi E (1985). "Cloning of the human myoglobin gene". Gene 33 (3): 241–9. doi:10.1016/0378-1119(85)90231-8. PMID 2989088.
- ^ McGee, H (2004). On Food and Cooking: The Science and Lore of the Kitchen. New York: Scribner. p. 148. ISBN 0-684-80001-2.
- ^ Minneapolis Star Tribune, Nov. 14, 2007 http://www.startribune.com/10223/story/1548852.html
- ^ Minneapolis Star Tribune, October 31, 2007 http://www.startribune.com/535/story/1518775.html
- ^ Toshio Naka, Daryl Jones, Ian Baldwin, Nigel Fealy, Samantha Bates, Hermann Goehl, Stanislao Morgera, Hans H. Neumayer and Rinaldo Bellomo (2005). "Myoglobin clearance by super high-flux hemofiltration in a case of severe rhabdomyolysis: a case report". Critical Care 9 (2): R90–5. doi:10.1186/cc3034. PMC 1175920. PMID 15774055. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1175920.
- ^ M. Weber, M. Rau, K. Madlener, A. Elsaesser, D. Bankovic, V. Mitrovic and C. Hamm (2005). "Diagnostic utility of new immunoassays for the cardiac markers cTnI, myoglobin and CK-MB mass". Clinical Biochemistry 38 (11): 1027–30. doi:10.1016/j.clinbiochem.2005.07.011. PMID 16125162.
- ^ J. P. Collman, J. I. Brauman, T. R. Halbert, and K. S. Suslick (1976). "Nature of Oxygen and Carbon Monoxide Binding to Metalloporphyrins and Heme Proteins". Proceedings of the National Academy of Sciences of the United States of America 73 (10): 3333–7. doi:10.1073/pnas.73.10.3333. PMC 431107. PMID 1068445. http://www.pnas.org/cgi/content/abstract/73/10/3333.
Further reading
- J. P. Collman, R. Boulatov, C. J. Sunderland and L. Fu (2004). "Functional Analogues of Cytochrome c Oxidase, Myoglobin, and Hemoglobin". Chem. Rev. 104 (2): 561–588. doi:10.1021/cr0206059. PMID 14871135.
- Reeder, BJ; Svistunenko DA, Cooper CE, Wilson MT (December 2004). "The radical and redox chemistry of myoglobin and hemoglobin: from in vitro studies to human pathology". Antioxid Redox Signal 6 (6): 954–66. doi:10.1089/ars.2004.6.954. PMID 15548893.
- Schlieper, G; Kim JH, Molojavyi A, Jacoby C, Laussmann T, Flogel U, Godecke A, Schrader J (April 2004). "Adaptation of the myoglobin knockout mouse to hypoxic stress". Am J Physiol Regul Integr Comp Physiol 286 (4): R786–92. doi:10.1152/ajpregu.00043.2003. PMID 14656764.
- Takano, T (1977). "Structure of myoglobin refined at 2-0 A resolution. II. Structure of deoxymyoglobin from sperm whale". J. Mol. Biol. 110 (3): 569–584. doi:10.1016/S0022-2836(77)80112-5. PMID 845960.
- Roy, A; Sen S, Chakraborti AS (February 2004). "In vitro nonenzymatic glycation enhances the role of myoglobin as a source of oxidative stress". Free Radic Res. 38 (2): 139–46. doi:10.1080/10715160310001638038. PMID 15104207.
- Stewart, JM; Blakely JA, Karpowicz PA, Kalanxhi E, Thatcher BJ, Martin BM (March 2004). "Unusually weak oxygen binding, physical properties, partial sequence, autoxidation rate and a potential phosphorylation site of beluga whale (Delphinapterus leucas) myoglobin". Comp Biochem Physiol B Biochem Mol Biol 137 (3): 401–12. doi:10.1016/j.cbpc.2004.01.007. PMID 15050527.
- Wu, G; Wainwright LM, Poole RK (2003). "Microbial globins". Adv Microb Physiol 47: 255–310. doi:10.1016/S0065-2911(03)47005-7. PMID 14560666.
External links
- The Myoglobin Protein
- Protein Database featured molecule
- Online 'Mendelian Inheritance in Man' (OMIM) 160000 human genetics
- Which Cut Is Older? (It's a Trick Question) New York Times, February 21, 2006 article regarding meat industry use of carbon monoxide to keep meat looking red.
- Stores React to Meat Reports New York Times, March 1, 2006 article on the use of carbon monoxide to make meat appear fresh.
Proteins: hemeproteins Globins SubunitsTetramersstages of development: HbA (α2β2) · HbA2 (α2δ2) · HbF/Fetal (α2γ2) · HbE Gower 2 (α2ε2) · HbE Gower 1 (ζ2ε2)
pathology: HbH (β4) · Barts (γ4) · HbS (α2βS2) · HbC (α2βC2) · HbE (α2βE2)CompoundsOther humanNonhumanOtherOther see also disorders of globin and globulin proteinsMedical test: Serology, reference range: Clinical biochemistry blood tests (including BMP, CMP) (CPT 82000-84999) Fluid/electrolytes electrolytes (Na+/K+, Cl-/HCO3-) · renal function, BUN-to-creatinine ratio (BUN/Creatinine) · Ca
derived values: Plasma osmolality · Serum osmolal gapAcid-base Nutrition Iron tests: Transferrin saturation = Serum iron / Total iron-binding capacity; Ferritin · Transferrin · Transferrin receptorEndocrine ACTH stimulation test · Thyroid function tests (TSH)
Blood sugar: Glucose test · C-peptide · Fructosamine · Glycated hemoglobinMetabolic Cardiovascular Digestive Liver function tests: protein tests (Human serum albumin, Serum total protein) · ALP · transaminases (ALT, AST, AST/ALT ratio) · Bilirubin (Unconjugated, Conjugated)
Amylase · Lipase (Pancreatic lipase)PDB gallery 1m6c: V68N MYOGLOBIN WITH CO1m6m: V68N MET MYOGLOBIN1mdn: WILD TYPE MYOGLOBIN WITH CO1mnh: INTERACTIONS AMONG RESIDUES CD3, E7, E10 AND E11 IN MYOGLOBINS: ATTEMPTS TO SIMULATE THE O2 AND CO BINDING PROPERTIES OF APLYSIA MYOGLOBIN1mni: ALTERATION OF AXIAL COORDINATION BY PROTEIN ENGINEERING IN MYOGLOBIN. BIS-IMIDAZOLE LIGATION IN THE HIS64-->VAL(SLASH)VAL68-->HIS DOUBLE MUTANT1mnj: INTERACTIONS AMONG RESIDUES CD3, E7, E10 AND E11 IN MYOGLOBINS: ATTEMPTS TO SIMULATE THE O2 AND CO BINDING PROPERTIES OF APLYSIA MYOGLOBIN1mnk: INTERACTIONS AMONG RESIDUES CD3, E7, E10 AND E11 IN MYOGLOBINS: ATTEMPTS TO SIMULATE THE O2 AND CO BINDING PROPERTIES OF APLYSIA MYOGLOBIN1mno: V68N MYOGLOBIN OXY FORM1mwc: WILD TYPE MYOGLOBIN WITH CO1mwd: WILD TYPE DEOXY MYOGLOBIN1myg: HIGH RESOLUTION X-RAY STRUCTURES OF PIG METMYOGLOBIN AND TWO CD3 MUTANTS MB(LYS45-> ARG) AND MB(LYS45-> SER)1myh: HIGH RESOLUTION X-RAY STRUCTURES OF PIG METMYOGLOBIN AND TWO CD3 MUTANTS MB(LYS45-> ARG) AND MB(LYS45-> SER)1myi: HIGH RESOLUTION X-RAY STRUCTURES OF PIG METMYOGLOBIN AND TWO CD3 MUTANTS MB(LYS45-> ARG) AND MB(LYS45-> SER)1myj: DISTAL POLARITY IN LIGAND BINDING TO MYOGLOBIN: STRUCTURAL AND FUNCTIONAL CHARACTERIZATION OF A THREONINE68(E11) MUTANT1pmb: THE DETERMINATION OF THE CRYSTAL STRUCTURE OF RECOMBINANT PIG MYOGLOBIN BY MOLECULAR REPLACEMENT AND ITS REFINEMENT1yca: DISTAL POCKET POLARITY IN LIGAND BINDING TO MYOGLOBIN: DEOXY AND CARBONMONOXY FORMS OF A THREONINE68 (E11) MUTANT INVESTIGATED BY X-RAY CRYSTALLOGRAPHY AND INFRARED SPECTROSCOPY1ycb: DISTAL POCKET POLARITY IN LIGAND BINDING TO MYOGLOBIN: DEOXY AND CARBONMONOXY FORMS OF A THREONINE68 (E11) MUTANT INVESTIGATED BY X-RAY CRYSTALLOGRAPHY AND INFRARED SPECTROSCOPY2mm1: X-RAY CRYSTAL STRUCTURE OF A RECOMBINANT HUMAN MYOGLOBIN MUTANT AT 2.8 ANGSTROMS RESOLUTIONCategories:- Human proteins
- Hemoproteins
Wikimedia Foundation. 2010.