- Chlorocruorin
-
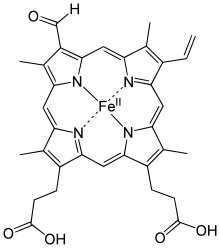
Chlorocruorin is an oxygen-binding hemeprotein present in the blood plasma of many annelids, particularly certain marine polychaetes.[1][2][3] Its affinity for oxygen is weaker than that of most hemoglobins. A dichromatic compound, chlorocruorin is noted for appearing green in dilute solutions, though it appears light red when found in concentrated solutions.[4][5][6]
Its structure is very similar to erythrocruorin (which is likewise very similar to multiple subunits of myoglobin) and it contains many 16-17kDa myoglobin-like subunits arranged in a giant complex of over a hundred subunits with interlinking proteins as well with a total weight exceeding 3600kDa. Because of its giant macromolecule structure, chlorocruorin is free floating in blood. The only significant difference between chlorocruorin and erythrocruorin is that chlorocruorin carries an abnormal heme group structure.[7] This enormous macromolecule is typically found free floating in the plasma, and not contained within red blood cells.[7][8]
References
- ^ H. Munro Fox (1 April 1933). "The Blood Circulation of Animals Possessing Chlorocruorin". Proceedings of the Royal Society of London (Series B, Biological Sciences) 112 (779): 479–495. doi:10.1098/rspb.1938.0042. JSTOR 81599.
- ^ R. F. Ewer, H. Munro Fox (9 August 1940). "On the Function of Chlorocruorin". Proceedings of the Royal Society of London (Series B, Biological Sciences) 129 (855): 137–153. doi:10.1098/rspb.1940.0033. JSTOR 82389.
- ^ D.W. Ewer (1941). "The blood systems of Sabella and Spirographis". Quarterly Journal of Microscopical Science 82 (s2): 587–619. http://jcs.biologists.org/cgi/reprint/s2-82/328/587. Retrieved 1 May 2010.
- ^ H. Munro Fox (1 February 1926). "Chlorocruorin: A Pigment Allied to Haemoglobin". Proceedings of the Royal Society of London (Series B, Biological Sciences) 99 (696): 199–220. doi:10.1098/rspb.1926.0008. JSTOR 81088.
- ^ H. Munro Fox (1 September 1932). "The Oxygen Affinity of Chlorocruorin". Proceedings of the Royal Society of London (Series B, Biological Sciences) 111 (772): 356–363. doi:10.1098/rspb.1932.0060. JSTOR 81716.
- ^ H. Munro Fox (19 October 1949). "On Chlorocruorin and Haemoglobin". Proceedings of the Royal Society of London (Series B, Biological Sciences) 136 (884): 378–388. doi:10.1098/rspb.1949.0031. JSTOR 82565.
- ^ a b Pallavicini A, Negrisolo E, Barbato R, et al. (July 2001). "The primary structure of globin and linker chains from the chlorocruorin of the polychaete Sabella spallanzanii". J. Biol. Chem. 276 (28): 26384–90. doi:10.1074/jbc.M006939200. PMID 11294828. http://www.jbc.org/cgi/pmidlookup?view=long&pmid=11294828.
- ^ Lamy JN, Green BN, Toulmond A, Wall JS, Weber RE, Vinogradov SN (19 December 1996). "Giant Hexagonal Bilayer Hemoglobins". Chem Rev 96 (8): 3113–3124. doi:10.1021/cr9600058. PMID 11848854.
External links
Proteins: hemeproteins Globins SubunitsTetramersstages of development: HbA (α2β2) · HbA2 (α2δ2) · HbF/Fetal (α2γ2) · HbE Gower 2 (α2ε2) · HbE Gower 1 (ζ2ε2)
pathology: HbH (β4) · Barts (γ4) · HbS (α2βS2) · HbC (α2βC2) · HbE (α2βE2)CompoundsOther humanNonhumanChlorocruorin · ErythrocruorinOtherhuman: Myoglobin (Metmyoglobin) · Neuroglobin · Cytoglobin
plant: LeghemoglobinOther see also disorders of globin and globulin proteins 
This annelid-related article is a stub. You can help Wikipedia by expanding it. This protein-related article is a stub. You can help Wikipedia by expanding it.