- Cytochrome b
-
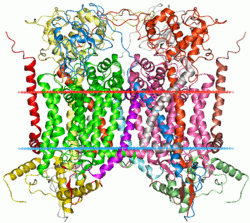
Mitochondrial cytochrome bc1 complex Identifiers Symbol Cytochrom_B_N Pfam PF00033 InterPro IPR005797 PROSITE PDOC00171 SCOP 3bcc TCDB 3.D.3 OPM family 3 OPM protein 1bcc Available protein structures: Pfam structures PDB RCSB PDB; PDBe PDBsum structure summary Cytochrome B C-terminal domain 
cytochrome bc1 complex from chicken Identifiers Symbol Cytochrom_B_C Pfam PF00032 InterPro IPR005798 PROSITE PDOC00171 SCOP 1bcc TCDB 3.D.3 Available protein structures: Pfam structures PDB RCSB PDB; PDBe PDBsum structure summary Cytochrome b/b6 is the main subunit of transmembrane cytochrome bc1 and b6f complexes.[1][2] In addition, it commonly refers to a region of mtDNA used for population genetics and phylogenetics.
Contents
Function
In the mitochondrion of eukaryotes and in aerobic prokaryotes, cytochrome b is a component of respiratory chain complex III (EC 1.10.2.2) - also known as the bc1 complex or ubiquinol-cytochrome c reductase. In plant chloroplasts and cyanobacteria, there is an analogous protein, cytochrome b6, a component of the plastoquinone-plastocyanin reductase (EC 1.10.99.1), also known as the b6f complex. These complexes are involved in electron transport and the generation of ATP and thus play a vital role in the cell.
Structure
Cytochrome b/b6[3][4] is an integral membrane protein of approximately 400 amino acid residues that probably has 8 transmembrane segments. In plants and cyanobacteria, cytochrome b6 consists of two subunits encoded by the petB and petD genes. Cytochrome b/b6 non-covalently binds two heme groups, known as b562 and b566. Four conserved histidine residues are postulated to be the ligands of the iron atoms of these two heme groups.
Use in phylogenetics
Cytochrome b is commonly used to determine phylogenetic relationships between organisms due to its sequence variability. It is considered to be most useful in determining relationships within families and genera. Comparative studies involving cytochrome b have resulted in new classification schemes and have been used to assign newly described species to a genus, as well as deepen the understanding of evolutionary relationships.[5]
Clinical significance
Mutations in cytochrome b primarily result in exercise intolerance in human patients; though more rare, severe multi-system pathologies have also been reported.[6]
Single-point mutations in cytochrome b of Plasdmodium falciparum and P. berghei are associated with resistance to the anti-malarial drug atovaquone.[7]
Human genes
Human genes encoding cytochrome b proteins include:
- CYB5A – cytochrome b5 type A (microsomal)
- CYB5B – cytochrome b5 type B (outer mitochondrial membrane)
- CYBASC3 – cytochrome b, ascorbate dependent 3
- MT-CYB – mitochondrially encoded cytochrome b
References
- ^ Howell N (August 1989). "Evolutionary conservation of protein regions in the protonmotive cytochrome b and their possible roles in redox catalysis". J. Mol. Evol. 29 (2): 157–69. doi:10.1007/BF02100114. PMID 2509716.
- ^ Esposti MD, De Vries S, Crimi M, Ghelli A, Patarnello T, Meyer A (July 1993). "Mitochondrial cytochrome b: evolution and structure of the protein". Biochim. Biophys. Acta 1143 (3): 243–71. doi:10.1016/0005-2728(93)90197-N. PMID 8329437.
- ^ Howell N (1989). "Evolutionary conservation of protein regions in the protonmotive cytochrome b and their possible roles in redox catalysis". J. Mol. Evol. 29 (2): 157–169. doi:10.1007/BF02100114. PMID 2509716.
- ^ Esposti MD, Crimi M, Ghelli A, Patarnello T, Meyer A, De Vries S (1993). "Mitochondrial cytochrome b: evolution and structure of the protein". Biochim. Biophys. Acta 1143 (3): 243–271. doi:10.1016/0005-2728(93)90197-N. PMID 8329437.
- ^ Castresana, J. (2001). "Cytochrome b Phylogeny and the Taxonomy of Great Apes and Mammals". Molecular Biology and Evolution 18 (4): 465–471. PMID 11264397. http://mbe.oxfordjournals.org/cgi/reprint/18/4/465.
- ^ Blakely EL, Mitchell AL, Fisher N, Meunier B, Nijtmans LG, Schaefer AM, Jackson MJ, Turnbull DM, Taylor RW (July 2005). "A mitochondrial cytochrome b mutation causing severe respiratory chain enzyme deficiency in humans and yeast". FEBS J. 272 (14): 3583–92. doi:10.1111/j.1742-4658.2005.04779.x. PMID 16008558.
- ^ Siregar JE, Syafruddin D, Matsuoka H, Kita K, Marzuki S (June 2008). "Mutation underlying resistance of Plasmodium berghei to atovaquone in the quinone binding domain 2 (Qo(2)) of the cytochrome b gene". Parasitology International 57 (2): 229–32. doi:10.1016/j.parint.2007.12.002. PMID 18248769.
External links
Proteins: hemeproteins Globins SubunitsTetramersstages of development: HbA (α2β2) · HbA2 (α2δ2) · HbF/Fetal (α2γ2) · HbE Gower 2 (α2ε2) · HbE Gower 1 (ζ2ε2)
pathology: HbH (β4) · Barts (γ4) · HbS (α2βS2) · HbC (α2βC2) · HbE (α2βE2)CompoundsOther humanNonhumanOtherhuman: Myoglobin (Metmyoglobin) · Neuroglobin · Cytoglobin
plant: LeghemoglobinOther see also disorders of globin and globulin proteins
This membrane protein-related article is a stub. You can help Wikipedia by expanding it.
