- 2,5-Dimethoxy-4-amylamphetamine
-
2,5-Dimethoxy-4-amylamphetamine 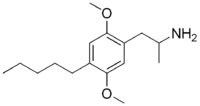 2-(2,5-Dimethoxy-4-pentyl-phenyl)-1-methyl-ethylamineOther names2,5-Dimethoxy-4-amyl-amphetamine;
2-(2,5-Dimethoxy-4-pentyl-phenyl)-1-methyl-ethylamineOther names2,5-Dimethoxy-4-amyl-amphetamine;
2,5-Dimethoxy-4-amyl-1-ethyl-(alpha-methyl)amineIdentifiers Abbreviations DOAM CAS number 63779-90-8 ChemSpider 10440619 
ChEMBL CHEMBL161416 
Jmol-3D images Image 1
Image 2- CCCCCC1=CC(OC)=C(CC(C)N)C=C1OC
COc1cc(CCCCC)c(cc1CC(C)N)OC
Properties Molecular formula C16H27NO2 Molar mass 265.39 g/mol  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Dimethoxy-4-amylamphetamine (DOAM) is a lesser-known psychedelic drug and a substituted amphetamine. DOAM was first synthesized by Alexander Shulgin. In his book PiHKAL (Phenethylamines i Have Known And Loved), the minimum dosage is listed as 10 mg, and the duration is unknown. DOAM produces a bare threshold and tenseness. As the 4-alkyl chain length is increased from shorter homologues such as DOM, DOET and DOPR which are all potent hallucinogens, the 5-HT2 binding affinity increases, rising to a maximum with the 4-(n-hexyl) derivative before falling again with even longer chains, but compounds with chain length longer than n-propyl, or with other bulky groups such as isopropyl, t-butyl or γ-phenylpropyl at the 4- position, fail to substitute for hallucinogens in animals or produce hallucinogenic effects in humans, suggesting these have low efficacy and are thus antagonists or weak partial agonists at the 5-HT2A receptor.[1][2][3]
References
- ^ Shulgin AT, Dyer DC (December 1975). "Psychotomimetic phenylisopropylamines. 5. 4-Alkyl-2,5-dimethoxyphenylisopropylamines". Journal of Medicinal Chemistry 18 (12): 1201–4. doi:10.1021/jm00246a006. PMID 1195275.
- ^ Seggel MR, Yousif MY, Lyon RA, Titeler M, Roth BL, Suba EA, Glennon RA (March 1990). "A structure-affinity study of the binding of 4-substituted analogues of 1-(2,5-dimethoxyphenyl)-2-aminopropane at 5-HT2 serotonin receptors". Journal of Medicinal Chemistry 33 (3): 1032–6. PMID 2308135.
- ^ Dowd CS, Herrick-Davis K, Egan C, DuPre A, Smith C, Teitler M, Glennon RA (August 2000). "1-[4-(3-Phenylalkyl)phenyl]-2-aminopropanes as 5-HT(2A) partial agonists". Journal of Medicinal Chemistry 43 (16): 3074–84. doi:10.1021/jm9906062. PMID 10956215.
See also
- 2,5-Dimethoxy-4-Substituted Amphetamines
External links

This psychoactive drug-related article is a stub. You can help Wikipedia by expanding it. - CCCCCC1=CC(OC)=C(CC(C)N)C=C1OC
