- Cichlid
-
Cichlids 
Common freshwater angelfish,
Pterophyllum scalareScientific classification Kingdom: Animalia Phylum: Chordata Class: Actinopterygii Order: Perciformes Suborder: Labroidei Family: Cichlidae
Heckel, 1840Subfamilies Astronotinae
Cichlasomatinae
Cichlinae
Etroplinae
Geophaginae
Heterochromidinae
Paratilapiinae
Pseudocrenilabrinae
Ptychochrominae
Retroculinae
For genera, see below.Cichlids (
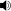 /ˈsɪklɨd/) are fishes from the family Cichlidae in the order Perciformes. Cichlids are members of a group known as the Labroidei along with the wrasses (Labridae), damselfish (Pomacentridae), and surfperches (Embiotocidae).[1] This family is both large and diverse. At least 1,300 species have been scientifically described,[2] making it one of the largest vertebrate families. New species are discovered annually, and many species remain undescribed. The actual number of species is therefore unknown, with estimates varying between 2,000 and 3,000.[3]
/ˈsɪklɨd/) are fishes from the family Cichlidae in the order Perciformes. Cichlids are members of a group known as the Labroidei along with the wrasses (Labridae), damselfish (Pomacentridae), and surfperches (Embiotocidae).[1] This family is both large and diverse. At least 1,300 species have been scientifically described,[2] making it one of the largest vertebrate families. New species are discovered annually, and many species remain undescribed. The actual number of species is therefore unknown, with estimates varying between 2,000 and 3,000.[3]Contents
Description
Cichlids span a wide range of body sizes, from species as small as 2.5 centimeters (0.98 in) in length (e.g., female Neolamprologus multifasciatus) to much larger species approaching 1 meter (3.3 ft) in length (e.g. Boulengerochromis and Cichla). As a group, cichlids exhibit a similar diversity of body shapes, ranging from strongly laterally compressed species (such as Altolamprologus, Pterophyllum, and Symphysodon) to species that are cylindrical and highly elongate (such as Julidochromis, Teleogramma, Teleocichla, Crenicichla, and Gobiocichla).[4] Generally, however, cichlids tend to be of medium size, ovate in shape and slightly laterally compressed, and generally similar to the North American sunfishes in morphology, behavior, and ecology.[5]
Many cichlids, particularly tilapia, are important food fishes, while others are valued game fish (e.g. Cichla species). The family also includes many familiar aquarium fish, including the angelfish, oscars, and discus.[4][6] Cichlids have the largest number of endangered species among vertebrate families, most in the haplochromine group.[7] Cichlids are particularly well known for having evolved rapidly into a large number of closely related but morphologically diverse species within large lakes, particularly Tanganyika, Victoria, Malawi, and Edward.[8][9] Their diversity in the African Great Lakes is important for the study of speciation in evolution.[10] Many cichlids that have been introduced into waters outside of their natural range have become nuisances, such as tilapia in the southern United States.[11]
Anatomy and appearance
Cichlids share a single key trait: the fusion of the lower pharyngeal bones into a single tooth-bearing structure. A complex set of muscles allows the upper and lower pharyngeal bones to be used as a second set of jaws for processing food, allowing a division of labor between the "true jaws" (mandibles) and the "pharyngeal jaws". Cichlids are efficient feeders that capture and process a very wide variety of food items. This is assumed to be one reason why they are so diverse.[4] Cichlids vary in body shape, ranging from compressed and disc-shaped (such as Symphysodon), to triangular (such as Pterophyllum), to elongate and cylindrical (such as Crenicichla).[12]
The features that distinguish them from the other Labroidei include:[2]
- A single nostril on each side of the forehead, instead of two.
- No bony shelf below the orbit of the eye.
- Division of the lateral line organ into two sections, one on the upper half of the flank and a second along the midline of the flank from about halfway along the body to the base of the tail (except for genera Teleogramma and Gobiocichla).
- A distinctively shaped otolith.
- The small intestine's left-side exit from the stomach instead of its right side as in other Labroidei.
Taxonomy
Kullander (1998) recognizes eight subfamilies of cichlids: the Astronotinae, Cichlasomatinae, Cichlinae, Etroplinae, Geophaginae, Heterochromidinae, Pseudocrenilabrinae, and Retroculinae.[13] Cichlid taxonomy is still debated, and classification of genera cannot yet be definitively given. A comprehensive system of assigning species to monophyletic genera is still lacking, and there is not complete agreement on what genera should be recognized in this family.[12]
As an example of the classification problems, Kullander[14] placed the African genus Heterochromis phylogenetically within neotropical cichlids, although later papers concluded otherwise. Other problems center upon the identity of the putative common ancestor for the Lake Victoria superflock, and the ancestral lineages of Tanganyikan cichlids.
Comparisons[15] between a morphologically-based phylogeny[16] and analyses of gene loci[17] produce differences at the genus level. There remains a consensus that the Cichlidae as a family is monophyletic.[18][19]
One problem that transformed cichlid taxonomy is related to dentition, which had been used as a classifying characteristic. In many cichlids, tooth shape changes with age, due to wear, and cannot be relied upon. Genome sequencing and other technologies transformed cichlid taxonomy.[20]
Range and habitat
Cichlids are the most species-rich non-Ostariophysan family in freshwaters worldwide. They are most diverse in Africa and South America. It is estimated that Africa alone hosts at least 1,600 species.[12] Central America and Mexico have approximately 120 species, as far north as the Rio Grande in southern Texas. Madagascar has its own distinctive species (Oxylapia, Paratilapia, Paretroplus, Ptychochromis, and Ptychochromoides), only distantly related to those on the African mainland.[2][22] Native cichlids are largely absent in Asia, except for nine species in Israel, Lebanon and Syria (Astatotilapia flaviijosephi, Oreochromis aureus, O. niloticus, Sarotherodon galilaeus , Tilapia zillii, and Tristramella spp.), one in Iran (Iranocichla), and three in India and Sri Lanka (Etroplus).[12] If disregarding Trinidad and Tobago (where the few native cichlids are members of genera that are widespread in the South American mainland), the three species from the genus Nandopsis are the only cichlids from the Antilles in the Caribbean, specifically Cuba and Hispaniola. Europe, Australia, Antarctica, and North America north of the Rio Grande drainage have no native cichlids, although in Florida, Mexico, Japan and northern Australia feral populations of cichlids have become established as exotics.[21][23][24][25][26][27][28]
Although most cichlids are found at relatively shallow depths, several exceptions do exist. These include species such as Alticorpus macrocleithrum and Pallidochromis tokolosh down to 150 meters (490 ft) below the surface in Lake Malawi,[29][30] and the whitish (non-pigmented) and blind Lamprologus lethops, which is believed to live as deep as 160 meters (520 ft) below the surface in the Congo River.[31]
Cichlids are less commonly found in brackish and saltwater habitats, though many species tolerate brackish water for extended periods; Cichlasoma urophthalmus, for example, is equally at home in freshwater marshes and mangrove swamps, and lives and breeds in saltwater environments such as the mangrove belts around barrier islands.[4] Several species of Tilapia, Sarotherodon, and Oreochromis are euryhaline and can disperse along brackish coastlines between rivers.[12] Only a few cichlids, however, inhabit primarily brackish or salt water, most notably Etroplus maculatus, Etroplus suratensis, and Sarotherodon melanotheron.[32] The perhaps most extreme habitats for cichlids are the warm hypersaline lakes where the members of the genera Alcolapia and Danakilia are found. Lake Abaeded in Eritrea encompasses the entire distribution of D. dinicolai, and its temperature ranges from 29 to 45 °C (84 to 113 °F).[33]
With the exception of the species from Cuba and Hispaniola, cichlids have not reached any oceanic island and have a predominantly Gondwanan distribution, showing the precise sister relationships predicted by vicariance: Africa-South America and India-Madagascar.[34] The dispersal hypothesis, in contrast, requires cichlids to have negotiated thousands of kilometers of open ocean between India and Madagascar without colonizing any other island or, for that matter, crossing the Mozambique Channel to Africa. Although the vast majority of Malagasy cichlids are entirely restricted to freshwater, Ptychochromis grandidieri and Paretroplus polyactis are commonly found in coastal brackish water and they are apparently salt tolerant,[35][36] as is also the case for Etroplus maculatus and E. suratensis from India and Sri Lanka.[37][38]
Ecology
Feeding
The bumblebee cichlid, Pseudotropheus crabro, is specialised in feeding on parasites from the catfish Bagrus meridionalis.[39]
Many cichlids are primarily herbivores feeding on algae (e.g. Petrochromis) and plants (e.g. Etroplus suratensis). Small animals, particularly invertebrates, are only a minor part of their diet.
Other cichlids are detritivores and eat all types of organic material; among these species are the tilapiines of the genera Oreochromis, Sarotherodon, and Tilapia.
Other cichlids are predatory and eat little or no plant matter. These include generalists that catch a variety of small animals, including other fishes and insect larvae (e.g. Pterophyllum), as well as variety of specialists. Trematocranus is a specialized snail-eater, while Pungu maclareni feeds on sponges. A number of cichlids feed on other fish, either entirely or in part. Crenicichla are stealth-predators that lunge from concealment at passing small fish, while Rhamphochromis are open water pursuit predators that chase down their prey.[40] Paedophagous cichlids such as the Caprichromis species eat other species' eggs or young, in some cases ramming the heads of mouthbrooding species to force them to disgorge their young.[39][41][42][43] Among the more unusual feeding strategies are those of Corematodus, Docimodus evelynae, Plecodus, Perissodus, and Genyochromis spp., which feed on scales and fins of other fishes, a behavior known as lepidophagy,[44][45][46] along with the death-mimicking behaviour of Nimbochromis and Parachromis species, which lay motionless, luring small fish to their side prior to ambush.[47][48]
This variety of feeding styles has helped cichlids to inhabit similarly varied habitats. Its pharyngeal teeth (teeth in the throat) afford cichlids so many "niche" feeding strategies, because the jaws pick and hold food, while the pharyngeal teeth crush the prey.
Reproduction
Cichlids have highly organized breeding activities.[12]
Brood care
All species show some form of parental care for both eggs and larvae, often nurturing free-swimming young until they are weeks or months old.
Communal parental care, where multiple monogamous pairs care for a mixed school of young have also been observed in multiple cichlid species, including Amphilophus citrinellus, Etroplus suratensis, and Tilapia rendalli.[49][50][51] Comparably, the fry of Neolamprologus brichardi, a species that commonly lives in large groups, are protected not only by the adults, but also by older juveniles from previous spawns.[52]
Several cichlids, including discus (Symphysodon spp.), some Amphilophus species, Etroplus and Uaru species feed their young with a skin secretion from mucous glands.[4][53]
Parental care falls into one of four categories:[53] substrate or open brooders, secretive cave brooders (also known as guarding speleophils[54]), and at least two types of mouthbrooders, ovophile mouthbrooders and larvophile mouthbrooders.[55]
Open brooding
Open or substrate brooding cichlids lay their eggs in the open, on rocks, leaves, or logs. Examples of open brooding cichlids include Pterophyllum, Symphysodon spp, and Anomalochromis thomasi. Male and female parents usually engage in differing brooding roles. Most commonly, the male patrols the pair's territory and repels intruders, while females fan water over the eggs, removing the infertile and leading the fry while foraging. However, both sexes are able to perform the full range of parenting behaviours.[55]
Cave brooding
Secretive cave spawning cichlids lay their eggs in caves, crevices, holes, or discarded mollusc shells, frequently attaching the eggs to the roof of the chamber. Examples include Pelvicachromis spp., Archocentrus spp, and Apistogramma spp.[53] Free-swimming fry and parents communicate in captivity and in the wild. Frequently this communication is based on body movements, such as shaking and pelvic fin flicking. In addition, open and cave brooding parents assist in finding food resources for their fry. Multiple neotropical cichlid species perform leaf-turning and fin-digging behaviors.[55]
Ovophile mouthbrooding
Ovophile mouthbrooders incubate their eggs in their mouths as soon as they are laid, and frequently mouthbrood free-swimming fry for several weeks. Examples include many Great Rift Valley lakes (Lake Malawi, Lake Tanganyika and Lake Victoria) endemics, e.g.: Maylandia, Pseudotropheus, and Tropheus, along with some South American cichlids such as Geophagus steindachneri.
Larvophile mouthbrooding
Larvophile mouthbrooders lay eggs in the open or in a cave and take the hatched larvae into the mouth. Examples include some variants of Geophagus altifrons, and some Aequidens, Gymnogeophagus, and Satanoperca.[4][53] Mouthbrooders, whether of eggs or larvae, are predominantly females. Exceptions that also involve the males include eretmodine cichlids (genera Spathodus, Eretmodus, and Tanganicodus), some Sarotherodon species, Chromidotilapia guentheri, and some Aequidens species.[4][55][56] Rare paternal mouthbrooding occurs, for example, in Sarotherodon melanotheron.[57] This method appears to have evolved independently in several groups of African cichlids.[12]
Mating
Cichlids mate either monogamously or polygamously.[4] The mating system of a given cichlid species is not consistently associated with its brooding system. For example, although most monogamous cichlids are not mouthbrooders, Chromidotilapia, Gymnogeophagus, Spathodus and Tanganicodus are all monogamous mouthbrooders. In contrast, numerous open or cave spawning cichlids are polygamous; examples include Apistogramma, Lamprologus, Nannacara and Pelvicachromis.[4][58]
Population status
In 2010, the International Union for Conservation of Nature classified 184 species as vulnerable, 52 as endangered, and 106 as critically endangered.[59] At present, the IUCN only lists Yssichromis sp. nov. "argens" as extinct in the wild, and six species are listed as entirely extinct, but it is acknowledged that many more possibly belong in these categories (for example, Haplochromis aelocephalus, H. apogonoides, H. dentex, H. dichrourus and numerous other members of the genus Haplochromis have not been seen since the 1980s, but are maintained as Critically Endangered in the small chance that tiny –but currently unknown– populations survive).[59]
Lake Victoria
Because of the introduced Nile perch (Lates niloticus) and water hyacinth, deforestation that led to water siltation, and overfishing, many Lake Victoria species have been wiped out or drastically reduced. By around 1980, lake fisheries yielded only 1 percent cichlids, a drastic decline from 80 percent in earlier years.[60]
About two-thirds of endemic cichlids (approximately 300 species), especially bottom feeders, became endangered or extinct. Some survivors have adapted by becoming smaller or hybridizing with other species.[60] Satellite lakes such as Lake Edward and Lake Kyoga have not been as strongly affected, however, and harbor an array of similar species.
Food and game fish
Although cichlids are mostly small- to medium-sized, many are notable as food and game fishes. With few thick rib bones and tasty flesh, artisan fishing is not uncommon in Central America and South America, as well as areas surrounding the African rift lakes.[60]
Tilapia
The most important food cichlids, however, are the tilapiines of North Africa. Fast growing, tolerant of stocking density, and adaptable, tilapiine species have been introduced and farmed extensively in many parts of Asia and are increasingly common aquaculture targets elsewhere.
Farmed tilapia production is about 1,500,000 tonnes (1,500,000 long tons; 1,700,000 short tons) annually with an estimated value of US$1.8 billion,[62] about equal to that of salmon and trout.
Unlike those carnivorous fish, tilapia can feed on algae or any plant-based food. This reduces the cost of tilapia farming, reduces fishing pressure on prey species, avoids concentrating toxins that accumulate at higher levels of the food chain and makes tilapia the preferred "aquatic chickens" of the trade.[60]
Game fish
Many large cichlids make good game fish. The strong, hard-fighting peacock bass (Cichla species) of South America is one of the most popular sportfish. It was introduced in many waters around the world. In Florida, this fish generates millions of hours of fishing and sportfishing revenue of more than US$8 million a year.[63] Other cichlids preferred by anglers include the Oscar, Mayan cichlid (Cichlasoma urophthalmus), and jaguar guapote (Parachromis managuensis).[63]
Aquarium fish
Since 1945, cichlids have become increasingly popular as aquarium fish.[4][53][55][64][65][66][67] Many cichlids are small to medium-sized, easy to feed with a range of prepared fish foods, breed readily, and practice brood care, making good aquarium fish.[53]
The most common species in hobbyist aquaria is Pterophyllum scalare from the Amazon River basin in tropical South America, known in the trade as the "angelfish". Other popular or readily available species include the oscar (Astronotus ocellatus), convict cichlid (Archocentrus nigrofasciatus) and discus (Symphysodon spp.).[4]
Cichlids can be kept in aquaria with other fish; however, many cichlids eat smaller fish.[53] Conversely, some cichlids, such as Apistogramma or Julidochromis spp., can be timid. In such cases the use of dither fish is recommended.[4]
Hybrids and selective breeding
 The "red Texas" cichlid is not a Texas cichlid (Herichthys cyanoguttatus) but an intergeneric hybrid of Herichthys and Amphilophus parents.
The "red Texas" cichlid is not a Texas cichlid (Herichthys cyanoguttatus) but an intergeneric hybrid of Herichthys and Amphilophus parents.
Some cichlids readily hybridize with related species, both in the wild and under artificial conditions.[68] Other groups of fishes, such as European cyprinids, also hybridize.[69] Unusually, cichlid hybrids have been put to extensive commercial use, in particular for aquaculture and aquaria.[6][70] The hybrid red strain of tilapia, for example, is often preferred in aquaculture for its rapid growth. Tilapia hybridization can produce all-male populations to control stock density or prevent reproduction in ponds.[6]
Aquarium hybrids
The most ubiquitous aquarium hybrid is perhaps the blood parrot cichlid which is a cross of several species, especially from genus Amphilophus. With a beak-shaped mouth, an abnormal spine, and an occasionally missing caudal fin (known as the "love heart" parrot cichlid), the fish is controversial among aquarists. Some have called blood parrot cichlids "the Frankenstein monster of the fish world."[71] Another notable hybrid, the flowerhorn cichlid, was very popular in some parts of Asia from 2001 until late 2003, and is believed to bring good luck to its owner.[72] The popularity of the flowerhorn cichlid declined in 2004.[73] Owners released many specimens into the rivers and canals of Malaysia and Singapore where they threaten endemic communities.[74]
Numerous cichlid species have been selectively bred to develop ornamental aquarium strains. The most intensive programs have involved angelfish and discus, and many mutations that affect both coloration and finnage are known.[4][75][76] Other cichlids have been bred for albino, leucistic, and xanthistic pigment mutations, including oscars, convicts and Pelvicachromis pulcher.[4][53] Both dominant and recessive pigment mutations have been observed.[10] In convict cichlids, for example, a leucistic coloration is recessively inherited,[77] while in Oreochromis niloticus niloticus red coloration is caused by a dominant inherited mutation.[78]
This selective breeding may have unintended consequences. For example, hybrid strains of Mikrogeophagus ramirezi have health and fertility problems.[79] Similarly, intentional inbreeding can cause physical abnormalities, such as the notched phenotype in angelfish.[80]
Genera
As of 2006, there were some 220 genera recognized and covered by FishBase:[2]
- Abactochromis Oliver & Arnegard 2010
- Acarichthys Eigenmann 1912
- Acaronia Myers 1940
- Aequidens Eigenmann & Bray 1894
- Allochromis Greenwood 1980
- Alticorpus Stauffer & McKaye 1988
- Altolamprologus Poll 1986
- Amatitlania Schmitter-Soto, 2007
- Amphilophus Agassiz, 1859
- Andinoacara Musilova, Z., Rican, O. & Novak, J., 2009
- Anomalochromis Greenwood 1985
- Apistogramma Regan 1913
- Apistogrammoides Meinken 1965
- Archocentrus Gill 1877
- Aristochromis Trewavas, 1935
- Astatoreochromis Pellegrin 1904
- Astatotilapia Pellegrin 1904
- Astronotus Swainson 1839
- Aulonocara Regan 1922
- Aulonocranus Regan 1920
- Australoheros Rican & Kullander 2006
- Baileychromis Poll 1986
- Bathybates Boulenger 1898
- Benitochromis Lamboj 2001
- Benthochromis Poll 1986
- Biotodoma Eigenmann & Kennedy 1903
- Biotoecus Eigenmann & Kennedy 1903
- Boulengerochromis Pellegrin 1904
- Buccochromis Eccles & Trewavas 1989
- Bujurquina Kullander 1986
- Callochromis Regan 1920
- Caprichromis Eccles & Trewavas 1989
- Caquetaia Fowler 1945
- Cardiopharynx Poll 1942
- Chaetobranchopsis Steindachner, 1875
- Chaetobranchus Heckel, 1840
- Chalinochromis Poll 1974
- Champsochromis Boulenger 1915
- Cheilochromis Eccles & Trewavas 1989
- Chetia Trewavas 1961
- Chilochromis Boulenger 1902
- Chilotilapia Boulenger 1908
- Chromidotilapia Boulenger 1898
- Cichla Bloch & Schneider 1801
- Cichlasoma Swainson 1839
- Cleithracara Kullander & Nijssen 1989
- Congochromis Stiassny & Schliewen 2007
- Copadichromis Eccles & Trewavas 1989
- Corematodus Boulenger 1897
- Crenicara Steindachner 1875
- Crenicichla Heckel 1840
- Cryptoheros Allgayer 2001
- Ctenochromis Pfeffer 1893
- Ctenopharynx Eccles & Trewavas 1989
- Cunningtonia Boulenger 1906
- Cyathochromis Trewavas 1935
- Cyathopharynx Regan 1920
- Cyclopharynx Poll 1948
- Cynotilapia Regan 1922
- Cyphotilapia Regan 1920
- Cyprichromis Scheuermann 1977
- Cyrtocara Boulenger 1902
- Danakilia Thys van den Audenaerde 1969
- Dicrossus Steindachner 1875
- Dimidiochromis Eccles & Trewavas 1989
- Diplotaxodon Trewavas 1935
- Divandu Lamboj & Snoeks 2000
- Docimodus Boulenger 1897
- Eclectochromis Eccles & Trewavas 1989
- Ectodus Boulenger 1898
- Eretmodus Boulenger 1898
- Etia Schliewen & Stiassny 2003
- Etroplus Cuvier 1830
- Exochochromis Eccles & Trewavas 1989
- Fossorochromis Eccles & Trewavas 1989
- Gaurochromis Greenwood 1980
- Genyochromis Trewavas 1935
- Geophagus Heckel 1840
- Gephyrochromis Boulenger 1901
- Gnathochromis Poll 1981
- Gobiocichla Kanazawa 1951
- Grammatotria Boulenger 1899
- Greenwoodochromis Poll 1983
- Guianacara Kullander & Nijssen 1989
- Gymnogeophagus Miranda Ribeiro 1918
- Haplochromis Hilgendorf 1888
- Haplotaxodon Boulenger 1906
- Hemibates Regan 1920
- Hemichromis Peters 1857
- Hemitaeniochromis Eccles & Trewavas 1989
- Hemitilapia Boulenger 1902
- Herichthys Baird & Girard 1854
- Heroina Kullander, 1996
- Heros Heckel, 1840
- Heterochromis Regan 1922
- Hoplarchus Kaup,1860
- Hoplotilapia Hilgendorf 1888
- Hypselecara Kullander 1986
- Hypsophrys Agassiz 1859
- Interochromis Yamaoka, Hori & Kuwamura 1988
- Iodotropheus Oliver & Loiselle 1972
- Iranocichla Coad 1982
- IvanacaraRomer & Hahn 2006[81]
- Julidochromis Boulenger 1898
- Katria Stiassny & Sparks 2006
- Konia Trewavas 1972
- Krobia Kullander & Nijssen 1989
- Labeotropheus Ahl 1926
- Labidochromis Trewavas 1935
- Labrochromis Greenwood 1980
- Laetacara Kullander 1986
- Lamprologus Schilthuis 1891
- Lepidiolamprologus Pellegrin 1904
- Lestradea Poll 1943
- Lethrinops Regan 1922
- Lichnochromis Trewavas 1935
- Limbochromis Greenwood 1987
- Limnochromis Regan 1920
- Limnotilapia Regan 1920
- Lipochromis Greenwood 1980
- Lithochromis Lippitsch & Seehausen 1998
- Lobochilotes Boulenger 1915
- Macropleurodus Regan 1922
- Maylandia Meyer & Foerster 1984
- (see also Metriaclima)
- Mazarunia Kullander 1990
- Mbipia Lippitsch & Seehausen 1998
- Mchenga Stauffer & Konings, 2006
- Melanochromis Trewavas, 1935
- Mesonauta Günther, 1862
- Microchromis Johnson 1975
- Mikrogeophagus Meulengracht-Madson 1968
- Myaka Trewavas 1972
- Mylacochromis Greenwood 1980
- Mylochromis Regan 1920
- Naevochromis Eccles & Trewavas 1989
- Nandopsis Gill, 1862
- Nannacara Regan 1905
- Nanochromis Pellegrin 1904
- Neochromis Regan 1920
- Neolamprologus Colombe & Allgayer 1985
- Nimbochromis Eccles & Trewavas 1989
- Nyassachromis Eccles & Trewavas 1989
- Ophthalmotilapia Pellegrin 1904
- Oreochromis Günther 1889
- Orthochromis Greenwood 1954
- Otopharynx Regan 1920
- Oxylapia Kiener & Maugé 1966
- Pallidochromis Turner 1994
- Parachromis Agassiz 1859
- Paracyprichromis Poll 1986
- Paralabidochromis Greenwood 1956
- Parananochromis Greenwood 1987
- Paraneetroplus Regan 1905
- Paratilapia Bleeker, 1868
- Paretroplus Bleeker, 1868
- Pelmatochromis Steindachner 1894
- Pelvicachromis Thys van den Audenaerde 1968
- Perissodus Boulenger 1898
- Petenia Günther, 1862
- Petrochromis Boulenger 1898
- Petrotilapia Trewavas 1935
- Pharyngochromis Greenwood 1979
- Placidochromis Eccles & Trewavas 1989
- Platytaeniodus Boulenger 1906
- Plecodus Boulenger 1898
- Protomelas Eccles & Trewavas 1989
- Pseudocrenilabrus Fowler 1934
- Pseudosimochromis Nelissen 1977
- Pseudotropheus Regan 1922
- Pterochromis Trewavas 1973
- Pterophyllum Heckel 1840
- Ptychochromis Steindachner 1880
- Ptychochromoides Kiener & Mauge 1966
- Pundamilia Seehausen & Lippitsch 1998
- Pungu Trewavas 1972
- Pyxichromis Greenwood 1980
- Reganochromis Whitley 1929
- Retroculus Eigenmann & Bray 1894
- Rhamphochromis Regan 1922
- Rocio Schmitter-Soto, 2007
- Sargochromis Regan 1920
- Sarotherodon Rppell 1852
- Satanoperca Günther, 1862
- Schubotzia Boulenger 1914
- Schwetzochromis Poll 1948
- Sciaenochromis Eccles & Trewavas 1989
- Serranochromis Regan 1920
- Simochromis Boulenger 1898
- Spathodus Boulenger 1900
- Steatocranus Boulenger 1899
- Stigmatochromis Eccles & Trewavas 1989
- Stomatepia Trewavas 1962
- Symphysodon Heckel 1840
- Taeniacara Myers 1935
- Taeniochromis Eccles & Trewavas 1989
- Taeniolethrinops Eccles & Trewavas 1989
- Tahuantinsuyoa Kullander 1991
- Tangachromis Poll 1981
- Tanganicodus Poll 1950
- Teleocichla Kullander 1988
- Teleogramma Boulenger 1899
- Telmatochromis Boulenger 1898
- Theraps Günther, 1862
- Thoracochromis Greenwood 1979
- Thorichthys Meek 1904
- Thysochromis Daget 1988
- Tilapia Smith, 1840 See also: Tilapiine cichlids
- Tomocichla Regan 1908
- Tramitichromis Eccles & Trewavas 1989
- Trematocara Boulenger 1899
- Trematocranus Trewavas 1935
- Triglachromis Poll & Thys van den Audenaerde 1974
- Tristramella Trewavas 1942
- Tropheus Boulenger 1898
- Tylochromis Regan 1920
- Tyrannochromis Eccles & Trewavas 1989
- Uaru Heckel 1840
- Variabilichromis Colombe & Allgayer 1985
- Vieja Fernandez-Yepez 1969
- Xenochromis Boulenger 1899
- Xenotilapia Boulenger 1899
- Yssichromis Greenwood 1980
Images of cichlids
- Main gallery: Cichlid images
-
The oscar (Astronotus ocellatus) is one of the most popular cichlids in the fishkeeping hobby.
-
The butterfly peacock bass (Cichla ocellaris) was introduced intentionally in Florida as gamefish.
-
The Nile tilapia (Oreochromis niloticus) is farmed extensively as food fish in many parts of the world.
-
Sexual dimorphism is common in cichlids. Shown here are a male (front, with egg spots) and a female (rear) Maylandia lombardoi.
-
A pair of blue rams (Mikrogeophagus ramirezi), male in front, female behind. Many cichlids form strong pair bonds while breeding.
-
Lake Malawi, Eastern Africa, is home to numerous cichild species including this Livingston's cichlid (Nimbochromis livingstonii).
-
A shell-brooding cichlid of the genus Lamprologus from Lake Tanganyika in East Africa
-
Pelvicachromis pulcher is a West African riverine cichlid, and part of the aquarists dwarf cichlid group.
-
Nannacara adoketa, a dwarf cichlid from Brazil
References
- ^ a b Stiassny, M.L.J.; Jensen, J.S. (1987). "Labroid intrarelationships revisited: morphological complexity, key innovations, and the study of comparative diversity". Bulletin of the Museum of Comparative Zoology, Harvard University 151: 269–319.
- ^ a b c d Froese, Rainer, and Daniel Pauly, eds. (2006). "Cichlidae" in FishBase. Nov 2006 version.
- ^ Stiassny, M., G. G. Teugels & C. D. Hopkins (2007). The Fresh and Brackish Water Fishes of Lower Guinea, West-Central Africa - Vol. 2. Musée Royal de l'Afrique Centrale. pp. 269. ISBN 9789074752213.
- ^ a b c d e f g h i j k l m n Loiselle, P.V. (1994). The Cichlid Aquarium. Tetra Press. ISBN 1-56465-146-0.
- ^ Helfman G., Collette B., & Facey D. (1997). The Diversity of Fishes. Blackwell Publishing, Inc.. pp. 256–257. ISBN 0-86542-256-7.
- ^ a b c Chapman, F. A. (1992) (PDF). Culture of Hybrid Tilapia: A Reference Profile. Circular 1051. University of Florida Institute of Food and Agricultural Sciences. http://edis.ifas.ufl.edu/pdffiles/FA/FA01200.pdf.
- ^ Reid, G. M. (December 1990). "Captive breeding for the conservation of cichlid fishes" (fee required). Journal of Fish Biology 37: 157. doi:10.1111/j.1095-8649.1990.tb05031.x. http://www.blackwell-synergy.com/doi/abs/10.1111/j.1095-8649.1990.tb05031.x?journalCode=jfb.
- ^ Salzburger W., Mack T., Verheyen E., Meyer A. (2005). "Out of Tanganyika: Genesis, explosive speciation, key-innovations and phylogeography of the haplochromine cichlid fishes" (PDF). BMC Evolutionary Biology 5 (17): 17. doi:10.1186/1471-2148-5-17. PMC 554777. PMID 15723698. http://www.biomedcentral.com/content/pdf/1471-2148-5-17.pdf.
- ^ Snoeks, J. (ed.) (2004). The cichlid diversity of Lake Malawi/Nyasa/Niassa: identification, distribution and taxonomy. Cichlid Press. ISBN 0-9668255-8-6.
- ^ a b Kornfield, Irv; Smith, Peter (November 2000). "African Cichlid Fishes: Model Systems for Evolutionary Biology". Annual Review of Ecology and Systematics 31: 163. doi:10.1146/annurev.ecolsys.31.1.163. http://arjournals.annualreviews.org/doi/abs/10.1146/annurev.ecolsys.31.1.163.
- ^ Gulf States Marine Fisheries Commission. "Fact sheet for Oreochromis mossambicus (Peters, 1852)". Gulf States Marine Fisheries Commission. http://nis.gsmfc.org/nis_factsheet2.php?toc_id=195. Retrieved 2006-10-20.
- ^ a b c d e f g Nelson, Joseph, S. (2006). Fishes of the World. John Wiley & Sons, Inc.. ISBN 0471250317.
- ^ Kullander, S.O. (1998). "A phylogeny and classification of the South American Cichlidae (Teleostei: Perciformes)". In L.R. Malabarba, R.E. Reis, R.P. Vari, Z.M. Lucena and C.A.S. Lucena (eds.). Phylogeny and classification of neotropical fishes. Porto Alegre: EDIPUCRS. pp. 461–498. ISBN 978-8574300351.
- ^ Phylogeny of major groups of cichlids
- ^ Multilocus Phylogeny of Cichlid Fishes (Pisces: Perciformes): Evolutionary Comparison of Microsatellite and Single-Copy Nuclear Loci by Streelman, Zardoya, Meyer and Karl (1998) (Mol. Biol. Evol. 15(7):798–808. 1998, paper available as PDF here
- ^ Stiassny, 1991
- ^ maximum-parsimony bootstrap consensus trees and majority-rule trees and other similar phylogenetic trees
- ^ From the various nuclear and mitochondrial DNA analyses in this and other papers
- ^ Further insights into the attractiveness of Cichlid taxonomy as a fertile area of research is given by the paper The species flocks of East African cichlid fishes: recent advances in molecular phylogenetics and population genetics by Salzburger and Meyer (Naturwissenschaften (2004) 91:277–290, paper available as PDF here), in which the advances made in the analysis of the phylogeny of the Lake Victoria superflock (among other East African Cichlids) is discussed in depth.
- ^ Highlighted by Dr Humphry Greenwood of the Natural History Museum, London, in a paper in 1977 (cited in TFH magazine, August 1977, with a follow up letter by Dr Greenwood in the November 1977 issue complaining about poor reportage of his work).
- ^ a b Koehn, J.D.; MacKenzie, R.F. (2004). "Priority management actions for alien freshwater fish species in Australia" (PDF). New Zealand Journal of Marine and Freshwater Research 38 (3): 457–472. doi:10.1080/00288330.2004.9517253. http://www.rsnz.org/publish/nzjmfr/2004/041.pdf. Retrieved 2007-04-19.[dead link]
- ^ Boruchowitz, D. E. (2006). Guide to Cichlids. T.F.H. Publications. ISBN 0-7938-0584-8.
- ^ ABC Far North Queensland. "Tilapia :: Far North Queensland". Archived from the original on 2007-10-17. http://web.archive.org/web/20071017061120/http://abc.net.au/farnorth/stories/s1313845.htm. Retrieved 2007-04-19.
- ^ Froese, R. and D. Pauly. Editors.. "Archocentrus nigrofasciatus, Convict cichlid". FishBase. http://filaman.uni-kiel.de/Summary/SpeciesSummary.php?id=3615. Retrieved 2007-03-29.
- ^ Yamamoto, M.N.; Tagawa, A.W. (2000). Hawai'i's native and exotic freshwater animals. Honolulu, Hawaii: Mutual Publishing. pp. 200.
- ^ Page, L.M.; Burr, B.M. (1991). A field guide to freshwater fishes of North America north of Mexico. Boston: Houghton Mifflin Company. pp. 432. ISBN 0395353076.
- ^ University of Southern Mississippi/College of Marine Sciences/Gulf Coast Research Laboratory (2005-08-03). "Fact Sheet for Tilapia zilli (Gervais, 1848)". Gulf States Marine Fisheries Commission. http://nis.gsmfc.org/nis_factsheet2.php?toc_id=200. Retrieved 2007-02-10.
- ^ Fuller, Pam L.; Leo G. Nico (2002-10-11). "Nonindigenous Fishes of Florida - With a Focus on South Florida". U.S. Department of the Interior, U.S. Geological Survey, Center for Coastal Geology. http://sofia.usgs.gov/sfrsf/rooms/species/invasive/focus/. Retrieved 2007-02-10.
- ^ Froese, Rainer, and Daniel Pauly, eds. (2006). "Alticorpus macrocleithrum" in FishBase. April 2006 version.
- ^ Froese, Rainer, and Daniel Pauly, eds. (2006). "Pallidochromis tokolosh" in FishBase. April 2006 version.
- ^ Norlander, Britt (April 20, 2009). Rough waters: one of the world's most turbulent rivers is home to a wide array of fish species. Now, large dams are threatening their future. Science World
- ^ Frank Schäfer (2005). Brackish-Water Fishes. Aqualog. ISBN 3-936027-82-X (English), ISBN 3-936027-81-1 (German).
- ^ Stiassny, de Marchi & Lamboj (2010). A new species of Danakilia (Teleostei, Cichlidae) from Lake Abaeded in the Danakil Depression of Eritrea (East Africa). Zootaxa 2690: 43–52.
- ^ Chakrabarty, P., Cichlid Biogeography: Comment and Review, Fish and Fisheries, Volume 5, Pages 97-119, 2004
- ^ Stiassny, M., and Sparks, J. S. (2006). Phylogeny and Taxonomic Revision of the endemic Malagasy genus Ptychochromis (Teleostei: Cichlidae), with the description of five new species and a diagnosis for Katria, new genus. American Museum Novitates 3535.
- ^ Sparks, J. S. (2008). Phylogeny of the Cichlid Subfamily Etroplinae and Taxonomic Revision of the Malagasy Cichlid Genus Paretroplus (Teleostei: Cichlidae). Bulletin of the American Museum of Natural History Number 314: 1-151
- ^ Froese, Rainer, and Daniel Pauly, eds. (2011). "Etroplus maculatus" in FishBase. July 2011 version.
- ^ Froese, Rainer, and Daniel Pauly, eds. (2011). "Etroplus suratensis" in FishBase. July 2011 version.
- ^ a b Ribbink, A.J.; Lewis, D.S.C. (1982). "Melanochromis crabro sp. nov.: a cichlid fish from Lake Malawi which feeds on ectoparasites and catfish eggs". Netherlands Journal of Zoology 32 (1): 72–87. doi:10.1163/002829682X00058..
- ^ Oliver, M.K. (1999-11-18). "Rhamphochromis esox". malawicichlids.com: The Cichlid Fishes of Lake Malawi. http://malawicichlids.com/mw08096.htm. Retrieved 2007-04-19.
- ^ McKaye, K.R.; Kocher, T. (1983). "Head ramming behaviour by three paedophagous cichlids in Lake Malawi, Africa". Animal Behaviour 31: 206. doi:10.1016/S0003-3472(83)80190-0.
- ^ Wilhelm, W. (1980). "The disputed feeding behavior of a paedophagous haplochromine cichlid (Pisces) observed and discussed". Behaviour 74 (3): 310. doi:10.1163/156853980X00528.
- ^ Konings, A. (2007). "Paedophagy in Malawi cichlids". Cichlid News 16: 28–32.
- ^ Trewavas, E. (1947). "An example of "mimicry" in fishes". Nature 160 (4056): 120. doi:10.1038/160120a0.
- ^ Eccles, D.H.; D.S.C. Lewis (1976). "A taxonomic study of the genus Docimodus Boulenger (Pisces, Cichlidae) a group of fishes with unusual feeding habits from Lake Malawi". Zoological Journal of the Linnean Society 58 (2): 165–172. doi:10.1111/j.1096-3642.1976.tb00826.x.
- ^ Nshombo, M. (1991). "Occasional egg-eating by the scale-eater Plecodus straeleni (Cichlidae) of Lake Tanganyika". Environmental Biology of Fishes 31 (2): 207–212. doi:10.1007/BF00001022.
- ^ Tobler, M. (2005). "Feigning death in the Central American cichlid Parachromis friedrichsthalii". Journal of Fish Biology 66 (3): 877. doi:10.1111/j.0022-1112.2005.00648.x.
- ^ McKaye, K.R. (1981). "Field observation on death feigning: a unique hunting behavior by the predatory cichlid, Haplochromis livingstoni, of Lake Malawi". Environmental Biology of Fishes 6 (3–4): 361–365. doi:10.1007/BF00005766.
- ^ McKaye, K.R.; N.M. McKaye (1977). "Communal Care and Kidnapping of Young by Parental Cichlids". Evolution 31 (3): 674–681. doi:10.2307/2407533. JSTOR 2407533.
- ^ Ward, J.A.; R.L. Wyman (1977). "Ethology and ecology of cichlid fishes of the genus Etroplus in Sri Lanka: preliminary findings". Environmental Biology of Fishes 2 (2): 137–145. doi:10.1007/BF00005369.
- ^ Ribbink, A.J.; A.C. Marsh, and B.A. Marsh (1981). "Nest-building and communal care of young by Tilapia rendalli dumeril (pisces, cichlidae) in Lake Malawi". Environmental Biology of Fishes 6 (2): 219–222. doi:10.1007/BF00002787.
- ^ Steeves, Greg. Neolamprologus brichardi. africancichlids.net. Accessed 2008-04-08
- ^ a b c d e f g h Riehl, Rüdiger. Editor.; Baensch, HA (1996. 5th Edn.). Aquarium Atlas. Germany: Tetra Press. ISBN 3-88244-050-3.
- ^ Balon, E.K. (1975). "Reproductive guilds of fishes: a proposal. and definition". Journal of the Fisheries Research Board of Canada 32 (6): 821–864. doi:10.1139/f75-110.
- ^ a b c d e Keenleyside, M.H.A. (1991). "Parental Care". Cichlid Fishes: behaviour, ecology and evolution. London: Chapman and Hall. pp. 191–208. ISBN 0412322005.
- ^ Coleman, R. (January 1999). "Mysterious mouthbrooders". Cichlid News: 32–33.
- ^ Kishida, M.; J.L. Specker (2000). "Paternal Mouthbrooding in the Black-Chinned Tilapia, Sarotherodon melanotheron (Pisces: Cichlidae): Changes in Gonadal Steroids and Potential for Vitellogenin Transfer to Larvae". Hormones and Behavior 37 (1): 40–48. doi:10.1006/hbeh.1999.1556. PMID 10712857.
- ^ Martin, E.; and M. Taborsky (1997). "Alternative male mating acttics in a cichlid, Pelvicachromis pulcher: a comparison of reproductive effort and success". Behavioral Ecology and Sociobiology 41 (5): 311–319. doi:10.1007/s002650050391.
- ^ a b IUCN 2010. IUCN Red List of Threatened Species. Version 2010.4. Downloaded on 26 April 2011.
- ^ a b c d Barlow, G. W. (2000). The Cichlid Fishes. Cambridge, MA: Perseus Publishing. ISBN 0-7382-0376-9.
- ^ Kaufman L (1996) Haplochromis latifasciata. In: IUCN 2006. 2006 IUCN Red List of Threatened Species.
- ^ De Silva, S.S; Subasinghe, R.P.; Bartley, D.M.; Lowther, A. :Tilapias as Alien Aquatics in Asia and the Pacific: A Review. FAO Fisheries Technical Paper. No. 453, 2004. [1]
- ^ a b Florida Fish and Wildlife Conservation Commission. "Fact Exotic Freshwater Fishes". http://floridafisheries.com/fishes/non-native.html. Retrieved 2007-03-18.
- ^ Sands D (1994) A fishkeepers guide to Central American cichlids. Tetra Press. Belgium pg 59-60.
- ^ Mills D (1993) Aquarium Fish Harper Collins ISBN 0-7322-5012-9
- ^ Konings A (1997) Back to nature guide to Malawi Cichlids Druckhaus Beltz, Germany. p. 13-23
- ^ Leibel WS (1993) A fishkeepers guide to South American cichlids. Tetra Press. Belgium pg 12-14.
- ^ Smith, P. F., Konings, A., and Kornfield I.: Hybrid origin of a cichlid population in Lake Malawi: implications for genetic variation and species diversity. Molecular Ecology 12, pp 2497–2504, 2003 [2]
- ^ Wood, A. B., and Jordan, D. R.: Fertility of roach × bream hybrids, Rutilus rutilus (L.) × Abramis brama (L.), and their identification. Journal of Fish Biology 30, pp 249-261, 1987
- ^ Matt Clarke. "Frequently asked questions on Parrot cichlids". Practical Fishkeeping. Archived from the original on 2007-09-26. http://web.archive.org/web/20070926235443/http://www.practicalfishkeeping.co.uk/pfk/pages/show_article.php?article_id=331. Retrieved 2006-10-20.
- ^ "It's The Frankenstein Monster Of The Fish World: The Blood Parrot!". AquaFriend.com. 2002-10-27. http://www.aquafriend.com/modules.php?op=modload&name=News&file=article&sid=66&mode=thread&order=0&thold=0. Retrieved 2006-12-05.
- ^ Arnold W (2003) Singapore's 'lucky' pet Luohan can outnumber people in homes. International Herald Tribune. July 1.
- ^ Crayfish the latest fad among pet lovers New Straits Times (Malaysia) (2004) 3rd September
- ^ Flower Horn: Joy in homes, a pest in rivers. New Straits Times (Malaysia) (2004) 14th July.
- ^ Norton J (1982) Angelfish genetics Freshwater And Marine Aquarium magazine 5:(4)
- ^ Koh TL, Khoo G, Fan LQ, Phang VPE (1999) Genetic diversity among wild forms and cultivated varieties of Discus (Symphysodon spp.) as revealed by Random Amplified Polymorphic DNA (RAPD) fingerprinting Aquaculture 173:485-497.
- ^ Itzkovich J, Rothbard S, Hulata G (1981) Inheritance of pink body colouration in cichlasoma nigrofasciatum Günther (Pisces, Cichlidae). Genetica 55: 15-16.
- ^ McAndrew CJ, Roubal FR, Roberts RJ, Bullock AM, McEwan IM (1988) The genetics and history of red, blond, and associated color variants in Oreochromis niloticus Genetica 76:172.
- ^ Linke H, Staeck L (1994) American cichlids I: Dwarf Cichlids. A handbook for their identification, care and breeding. Tetra Press. Germany. ISBN 1-56465-168-1
- ^ Norton J (1994) Notched - An Angelfish Deformity Freshwater And Marine Aquarium magazine 17:(3)
- ^ Römer, U. & Hahn, I. (2006). "Ivanacara gen. n. (Teleostei: Perciformes, Cichlasomatini): a new genus of cichlids from the Neotropis". In Römer, U.. Cichlid Atlas 2. pp. 1190–1197.
Further reading
- Barlow, G. W. (2000). The Cichlid fishes. Cambridge MA: Perseus Publishing.
- "Cichlidae". Integrated Taxonomic Information System. http://www.itis.gov/servlet/SingleRpt/SingleRpt?search_topic=TSN&search_value=169770.: National Museum of Natural History, Washington, D.C., 2004-05-11).
External links
- Cichlid at the Open Directory Project
- The Cichlid Fishes of Lake Malawi by Dr. Michael Oliver.
- Vision in Cichlids: Ecomorphology of vision in haplochromine cichlids of Lake Victoria by Dr. H.J. van der Meer.
Categories:- Cichlidae
- Labroidei
- Fishkeeping
Wikimedia Foundation. 2010.