- CDKN1B
-
Cyclin-dependent kinase inhibitor 1B is an enzyme that in humans is encoded by the CDKN1B gene.[1] It encodes a protein which belongs to the Cip/Kip family of cyclin dependent kinase (Cdk) inhibitor proteins. The encoded protein binds to and prevents the activation of cyclin E-CDK2 or cyclin D-CDK4 complexes, and thus controls the cell cycle progression at G1. It is often referred to as a cell cycle inhibitor protein because its major function is to stop or slow down the cell division cycle.
Contents
Biochemical function
The p27Kip1 gene has a DNA sequence similar to other members of the "Cip/Kip" family which include the p21Cip1/Waf1 and p57Kip2 genes. In addition to this structural similarity the "Cip/Kip" proteins share the functional characteristic of being able to bind several different classes of Cyclin and Cdk molecules. For example, p27Kip1 binds to cyclin D either alone, or when complexed to its catalytic subunit CDK4. In doing so p27Kip1 inhibits the catalytic activity of Cdk4, which means that it prevents Cdk4 from adding phosphate residues to its principal substrate, the retinoblastoma (pRb) protein. Increased levels of the p27Kip1 protein typically cause cells to arrest in the G1 phase of the cell cycle. Likewise, p27Kip1 is able to bind other Cdk proteins when complexed to cyclin subunits such as Cyclin E/Cdk2 and Cyclin A/Cdk2.
Regulation
In general, extracellular growth factors which prevent cell growth cause an increase in p27Kip1 levels inside a cell. For example, levels of p27Kip1 increase when Transforming Growth Factor β (TGF β) is present outside of epithelial cells causing a growth arrest.[2] In contrast interleukin 2 (IL-2) causes p27Kip1 levels to drop in T-lymphocytes. A mutation of this gene may lead to loss of control over the cell cycle leading to uncontrolled cellular proliferation.[3][4][5] Loss of p27 expression has been observed in metastatic canine mammary carcinomas.[6][7][8] Decreased TGF-beta signalling has been suggested to cause loss of p27 expression in this tumor type.[9]
A structured cis-regulatory element has been found in the 5' UTR of the P27 mRNA where it is thought to regulate translation relative to cell cycle progression.[10]
Interactions
CDKN1B has been shown to interact with SPDYA,[11] XPO1,[12][13] Cyclin-dependent kinase 2,[11][13][14][15][16] SKP2,[17][18][19] Cyclin-dependent kinase 4,[20][21] Grb2,[22] AKT1,[19] Cyclin D3,[20][23][24] Cyclin E1,[13][25] NUP50[26] and CKS1B.[17][18]
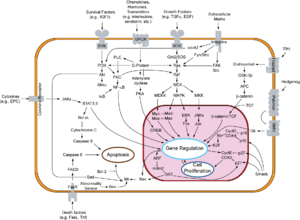 Overview of signal transduction pathways involved in apoptosis.
Overview of signal transduction pathways involved in apoptosis.
See also
- Sic1 (homologue in Saccharomyces cerevisiae)
References
- ^ Polyak K, Lee MH, Erdjument-Bromage H, Koff A, Roberts JM, Tempst P, Massague J (Aug 1994). "Cloning of p27Kip1, a cyclin-dependent kinase inhibitor and a potential mediator of extracellular antimitogenic signals". Cell 78 (1): 59–66. doi:10.1016/0092-8674(94)90572-X. PMID 8033212.
- ^ Toyoshima H, Hunter T (July 1994). "p27, a novel inhibitor of G1 cyclin-Cdk protein kinase activity, is related to p21". Cell 78 (1): 67–74. doi:10.1016/0092-8674(94)90573-8. PMID 8033213.
- ^ Fero ML, Rivkin M, Tasch M, Porter P, Carow CE, Firpo E, Polyak K, Tsai LH, Broudy V, Perlmutter RM, Kaushansky K, Roberts JM (May 1996). "A syndrome of multiorgan hyperplasia with features of gigantism, tumorigenesis, and female sterility in p27(Kip1)-deficient mice". Cell 85 (5): 733–44. doi:10.1016/S0092-8674(00)81239-8. PMID 8646781.
- ^ Kiyokawa H, Kineman RD, Manova-Todorova KO, Soares VC, Hoffman ES, Ono M, Khanam D, Hayday AC, Frohman LA, Koff A (May 1996). "Enhanced growth of mice lacking the cyclin-dependent kinase inhibitor function of p27(Kip1)". Cell 85 (5): 721–32. doi:10.1016/S0092-8674(00)81238-6. PMID 8646780.
- ^ Nakayama K, Ishida N, Shirane M, Inomata A, Inoue T, Shishido N, Horii I, Loh DY, Nakayama K (May 1996). "Mice lacking p27(Kip1) display increased body size, multiple organ hyperplasia, retinal dysplasia, and pituitary tumors". Cell 85 (5): 707–20. doi:10.1016/S0092-8674(00)81237-4. PMID 8646779.
- ^ Klopfleisch R, Gruber AD. (Jan 2009). "Differential expression of cell cycle regulators p21, p27 and p53 in metastasizing canine mammary adenocarcinomas versus normal mammary glands.". Res Vet Sci. 87 (1): 91–6. doi:10.1016/j.rvsc.2008.12.010. PMID 19185891.
- ^ Klopfleisch R, Schütze M, Gruber AD. (Sep 2010). "Loss of p27 expression in canine mammary tumors and their metastases.". Res Vet Sci. 88 (2): 300–3. doi:10.1016/j.rvsc.2009.08.007. PMID 19748645.
- ^ Klopfleisch R, von Euler H, Sarli G, Pinho SS, Gärtner F, Gruber AD. (2010). "Molecular Carcinogenesis of Canine Mammary Tumors: News From an Old Disease". Veterinary Pathology 228 (1): 98–116. doi:10.1177/0300985810390826. PMID 21149845.
- ^ Klopfleisch R, Schütze M, Gruber AD. (Oct 2009). "Downregulation of transforming growth factor β (TGFβ) and latent TGFβ binding protein (LTBP)-4 expression in late stage canine mammary tumours". Veterinary Journal 186 (3): 379–84. doi:10.1016/j.tvjl.2009.09.014. PMID 19836277.
- ^ Gopfert, U; Kullmann M, Hengst L (2003). "Cell cycle-dependent translation of p27 involves a responsive element in its 5'-UTR that overlaps with a uORF". Hum Mol Genet 12 (14): 1767–1779. doi:10.1093/hmg/ddg177. PMID 12837699.
- ^ a b Porter, Lisa A; Kong-Beltran Monica, Donoghue Daniel J (Sep. 2003). "Spy1 Interacts with p27Kip1 to Allow G1/S Progression". Mol. Biol. Cell (United States) 14 (9): 3664–74. doi:10.1091/mbc.E02-12-0820. ISSN 1059-1524. PMC 196558. PMID 12972555. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=196558.
- ^ Ishida, Noriko; Hara Taichi, Kamura Takumi, Yoshida Minoru, Nakayama Keiko, Nakayama Keiichi I (Apr. 2002). "Phosphorylation of p27Kip1 on serine 10 is required for its binding to CRM1 and nuclear export". J. Biol. Chem. (United States) 277 (17): 14355–8. doi:10.1074/jbc.C100762200. ISSN 0021-9258. PMID 11889117.
- ^ a b c Connor, Michael K; Kotchetkov Rouslan, Cariou Sandrine, Resch Ansgar, Lupetti Rafaella, Beniston Richard G, Melchior Frauke, Hengst Ludger, Slingerland Joyce M (Jan. 2003). "CRM1/Ran-Mediated Nuclear Export of p27Kip1 Involves a Nuclear Export Signal and Links p27 Export and Proteolysis". Mol. Biol. Cell (United States) 14 (1): 201–13. doi:10.1091/mbc.E02-06-0319. ISSN 1059-1524. PMC 140238. PMID 12529437. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=140238.
- ^ Youn, Cha-Kyung; Cho Hyun-Ju, Kim Soo-Hyun, Kim Hong-Beum, Kim Mi-Hwa, Chang In-Youb, Lee Jung-Sup, Chung Myung-Hee, Hahm Kyung-Soo, You Ho Jin (Feb. 2005). "Bcl-2 expression suppresses mismatch repair activity through inhibition of E2F transcriptional activity". Nat. Cell Biol. (England) 7 (2): 137–47. doi:10.1038/ncb1215. ISSN 1465-7392. PMID 15619620.
- ^ Law, Brian K; Chytil Anna, Dumont Nancy, Hamilton Elizabeth G, Waltner-Law Mary E, Aakre Mary E, Covington Cassondra, Moses Harold L (Dec. 2002). "Rapamycin Potentiates Transforming Growth Factor β-Induced Growth Arrest in Nontransformed, Oncogene-Transformed, and Human Cancer Cells". Mol. Cell. Biol. (United States) 22 (23): 8184–98. doi:10.1128/MCB.22.23.8184-8198.2002. ISSN 0270-7306. PMC 134072. PMID 12417722. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=134072.
- ^ Rosner, Margit; Hengstschläger Markus (Nov. 2004). "Tuberin binds p27 and negatively regulates its interaction with the SCF component Skp2". J. Biol. Chem. (United States) 279 (47): 48707–15. doi:10.1074/jbc.M405528200. ISSN 0021-9258. PMID 15355997.
- ^ a b Wang, Wei; Ungermannova Dana, Chen Lin, Liu Xuedong (Aug. 2003). "A negatively charged amino acid in Skp2 is required for Skp2-Cks1 interaction and ubiquitination of p27Kip1". J. Biol. Chem. (United States) 278 (34): 32390–6. doi:10.1074/jbc.M305241200. ISSN 0021-9258. PMID 12813041.
- ^ a b Sitry, Danielle; Seeliger Markus A, Ko Tun K, Ganoth Dvora, Breward Sadie E, Itzhaki Laura S, Pagano Michele, Hershko Avram (Nov. 2002). "Three different binding sites of Cks1 are required for p27-ubiquitin ligation". J. Biol. Chem. (United States) 277 (44): 42233–40. doi:10.1074/jbc.M205254200. ISSN 0021-9258. PMID 12140288.
- ^ a b Fujita, Naoya; Sato Saori, Katayama Kazuhiro, Tsuruo Takashi (Aug. 2002). "Akt-dependent phosphorylation of p27Kip1 promotes binding to 14-3-3 and cytoplasmic localization". J. Biol. Chem. (United States) 277 (32): 28706–13. doi:10.1074/jbc.M203668200. ISSN 0021-9258. PMID 12042314.
- ^ a b Lin, J; Jinno S, Okayama H (Apr. 2001). "Cdk6-cyclin D3 complex evades inhibition by inhibitor proteins and uniquely controls cell's proliferation competence". Oncogene (England) 20 (16): 2000–9. doi:10.1038/sj.onc.1204375. ISSN 0950-9232. PMID 11360184.
- ^ Cariou, S; Donovan J C, Flanagan W M, Milic A, Bhattacharya N, Slingerland J M (Aug. 2000). "Down-regulation of p21WAF1/CIP1 or p27Kip1 abrogates antiestrogen-mediated cell cycle arrest in human breast cancer cells". Proc. Natl. Acad. Sci. U.S.A. (UNITED STATES) 97 (16): 9042–6. doi:10.1073/pnas.160016897. ISSN 0027-8424. PMC 16818. PMID 10908655. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=16818.
- ^ Sugiyama, Y; Tomoda K, Tanaka T, Arata Y, Yoneda-Kato N, Kato J (Apr. 2001). "Direct binding of the signal-transducing adaptor Grb2 facilitates down-regulation of the cyclin-dependent kinase inhibitor p27Kip1". J. Biol. Chem. (United States) 276 (15): 12084–90. doi:10.1074/jbc.M010811200. ISSN 0021-9258. PMID 11278754.
- ^ Rual, Jean-François; Venkatesan Kavitha, Hao Tong, Hirozane-Kishikawa Tomoko, Dricot Amélie, Li Ning, Berriz Gabriel F, Gibbons Francis D, Dreze Matija, Ayivi-Guedehoussou Nono, Klitgord Niels, Simon Christophe, Boxem Mike, Milstein Stuart, Rosenberg Jennifer, Goldberg Debra S, Zhang Lan V, Wong Sharyl L, Franklin Giovanni, Li Siming, Albala Joanna S, Lim Janghoo, Fraughton Carlene, Llamosas Estelle, Cevik Sebiha, Bex Camille, Lamesch Philippe, Sikorski Robert S, Vandenhaute Jean, Zoghbi Huda Y, Smolyar Alex, Bosak Stephanie, Sequerra Reynaldo, Doucette-Stamm Lynn, Cusick Michael E, Hill David E, Roth Frederick P, Vidal Marc (Oct. 2005). "Towards a proteome-scale map of the human protein-protein interaction network". Nature (England) 437 (7062): 1173–8. doi:10.1038/nature04209. PMID 16189514.
- ^ Zhang, Q; Wang X, Wolgemuth D J (Jun. 1999). "Developmentally regulated expression of cyclin D3 and its potential in vivo interacting proteins during murine gametogenesis". Endocrinology (UNITED STATES) 140 (6): 2790–800. doi:10.1210/en.140.6.2790. ISSN 0013-7227. PMID 10342870.
- ^ Shanahan, F; Seghezzi W, Parry D, Mahony D, Lees E (Feb. 1999). "Cyclin E Associates with BAF155 and BRG1, Components of the Mammalian SWI-SNF Complex, and Alters the Ability of BRG1 To Induce Growth Arrest". Mol. Cell. Biol. (UNITED STATES) 19 (2): 1460–9. ISSN 0270-7306. PMC 116074. PMID 9891079. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=116074.
- ^ Smitherman, M; Lee K, Swanger J, Kapur R, Clurman B E (Aug. 2000). "Characterization and Targeted Disruption of Murine Nup50, a p27Kip1-Interacting Component of the Nuclear Pore Complex". Mol. Cell. Biol. (UNITED STATES) 20 (15): 5631–42. doi:10.1128/MCB.20.15.5631-5642.2000. ISSN 0270-7306. PMC 86029. PMID 10891500. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=86029.
Further reading
- Marone M, Bonanno G, Rutella S, et al. (2003). "Survival and cell cycle control in early hematopoiesis: role of bcl-2, and the cyclin dependent kinase inhibitors P27 and P21". Leuk. Lymphoma 43 (1): 51–7. doi:10.1080/10428190210195. PMID 11908736.
- Hirabayashi H (2003). "[P27 expression and survival in NSCLC]". Nippon Rinsho 60 Suppl 5: 263–6. PMID 12101669.
- Bloom J, Pagano M (2003). "Deregulated degradation of the cdk inhibitor p27 and malignant transformation". Semin. Cancer Biol. 13 (1): 41–7. doi:10.1016/S1044-579X(02)00098-6. PMID 12507555.
- Tokumoto M, Tsuruya K, Fukuda K, et al. (2003). "Parathyroid cell growth in patients with advanced secondary hyperparathyroidism: vitamin D receptor and cyclin-dependent kinase inhibitors, p21 and p27". Nephrol. Dial. Transplant. 18 Suppl 3: iii9–12. PMID 12771291.
- Drexler HC (2004). "The role of p27Kip1 in proteasome inhibitor induced apoptosis". Cell Cycle 2 (5): 438–41. PMID 12963837.
- Le XF, Pruefer F, Bast RC (2006). "HER2-targeting antibodies modulate the cyclin-dependent kinase inhibitor p27Kip1 via multiple signaling pathways". Cell Cycle 4 (1): 87–95. PMID 15611642.
- Belletti B, Nicoloso MS, Schiappacassi M, et al. (2005). "p27(kip1) functional regulation in human cancer: a potential target for therapeutic designs". Curr. Med. Chem. 12 (14): 1589–605. doi:10.2174/0929867054367149. PMID 16022660.
PDB gallery Cell cycle proteins Cyclin CDK CDK inhibitor P53 p63 p73 family Phases and
checkpointsOther cellular phasesB bsyn: dna (repl, cycl, reco, repr) · tscr (fact, tcrg, nucl, rnat, rept, ptts) · tltn (risu, pttl, nexn) · dnab, rnab/runp · stru (domn, 1°, 2°, 3°, 4°) Neoplasm: Tumor suppressor genes/proteins and Oncogenes/Proto-oncogenes Ligand Receptor TSP: CDH1TSP: PTCH1TSP: TGF beta receptor 2Intracellular signaling P+Ps ONCO: Beta-catenin · TSP: APCHippo signaling pathwayOther/unknownNucleus TSP: VHL · ONCO: CBL - MDM2Mitochondria Other/ungrouped M: NEO
tsoc, mrkr
tumr, epon, para
drug (L1i/1e/V03)
Categories:- Human proteins
- Cell cycle
- Tumor suppressor genes
Wikimedia Foundation. 2010.