- Terbinafine
-
Terbinafine 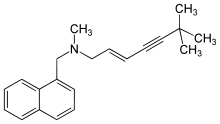
Systematic (IUPAC) name [(2E)-6,6-dimethylhept-2-en-4-yn-1-yl](methyl)(naphthalen-1-ylmethyl)amine Clinical data Trade names Lamisilat AHFS/Drugs.com monograph MedlinePlus a699061 Pregnancy cat. B Legal status ? Routes Oral, topical Pharmacokinetic data Bioavailability Readily absorbed: 70–90% Protein binding >99% Metabolism Hepatic Half-life Highly variable Identifiers CAS number 91161-71-6  78628-80-5
78628-80-5ATC code D01AE15 D01BA02 PubChem CID 1549008 DrugBank APRD00508 ChemSpider 1266005 
UNII G7RIW8S0XP 
KEGG D02375 
ChEMBL CHEMBL822 
Chemical data Formula C21H25N Mol. mass 291.43 g/mol SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)Terbinafine hydrochloride (Lamisil in Argentina, Australia, Belgium, Brazil, Canada, Chile, Finland, France, Germany, Greece, Hungary, Iceland, Ireland, Israel, Mexico, New Zealand, Romania, Russia, Slovenia, South Africa, Sweden, United Kingdom, United States and Venezuela, also sold under the name Corbinal and Terbisil in Turkey) is a synthetic allylamine antifungal from Novartis. It is highly lipophilic in nature and tends to accumulate in skin, nails, and fatty tissues. As a generic it is sold under the name Zabel in Australia. It is also available as a generic medication in the United States, United Kingdom, Belgium, Switzerland and Brazil.
In India, Terbinafine hydrochloride is available in topical form under the brand name Sebifin (Ranbaxy Labs), Zimig (GSK Pharma) and mycoCeaze (Progreś Laboratories).
MycoVa, developed by Apricus Biosciences, is a topical nail solution of terbinafine and DDAIP which has completed three Phase III studies for the treatment of onychomycosis.
Contents
Pharmacology
Terbinafine hydrochloride is a white fine crystalline powder that is freely soluble in methanol and dichloromethane, soluble in ethanol, and slightly soluble in water.
Like other allylamines, terbinafine inhibits ergosterol synthesis by inhibiting squalene epoxidase, an enzyme that is part of the fungal cell membrane synthesis pathway. Because terbinafine prevents conversion of squalene to lanosterol, ergosterol cannot be synthesized. This is thought to change cell membrane permeability, causing fungal cell lysis.
Indications
Terbinafine is mainly effective on the dermatophytes group of fungi.
As a 1% cream or powder it is used for superficial skin infections such as jock itch (Tinea cruris), athlete's foot (Tinea pedis) and other types of ringworm (Tinea corporis).
Oral 250mg tablets are often prescribed for the treatment of onychomycosis of the toenail or fingernail due to the dermatophyte Tinea unguium. Fungal nail infections are located deep under the nail in the cuticle to which topically applied treatments are unable to penetrate in sufficient amounts. The tablets may, rarely, cause hepatotoxicity, so patients are warned of this and may be monitored with liver function tests. Alternatives to oral administration have been studied. In 2009, results from a clinical study of a new formulation (terbinafine in Transfersomes, referred to as TDT-067) for topical treatment of onychomycosis were reported by Celtic Pharma.[1]
It has been found that terbinafine hydrochloride may induce or exacerbate subacute cutaneous lupus erythematosus. Persons with lupus erythematosus should first discuss possible risks with their doctor before initiation of therapy.[2]
FDA approval
The U.S. Food and Drug Administration has approved the first generic versions of prescription Lamisil (terbinafine hydrochloride) tablets. The remaining patent or exclusivity for Lamisil expired on June 30, 2007.
On September 28, 2007, the FDA stated that Lamisil (terbinafine hydrochloride, by Novartis AG) is a new treatment approved for use by children age 4 and up. The antifungal granules can be sprinkled on a child's food to treat ringworm of the scalp, Tinea capitis. [3]
Side effects
Many side effects and adverse drug reactions have been reported with terbinafine[4][5][6] possibly due to its extensive biodistribution and the often extended durations involved in anti-fungal treatment ( > 2 months ). The following is a comprehensive list of adverse events that are associated with terbinafine use:
Gastrointestinal Problems Diarrhea, constipation, nausea, sickness, fullness, abdominal pain, indigestion, dyspepsia, gastritis, cholestasis, flatulence, altered stool colour,abdominal muscular pain.
Central Nervous System / Neurological Problems Headaches, dizziness, vertigo, light-headedness, decreased concentration levels, paraesthesia (pins and needles)
Hepatic Problems Raised liver enzymes, liver inflammation (hepatitis), liver damage, liver failure
Immune System Problems Decreased white blood cell counts including pancytopenia, leukopenia, lymphopenia, thrombocytopenia, agranulocytosis, and neutropenia. Auto-immune reactions such as lupus erythematosus
Psychological Problems Depression, anxiety, insomnia, increased / unusual dream activity, malaise.
Sensory Problems Complete loss of taste (ageusia), decreased taste (hypogeusia) and distorted taste (dysgeusia) often involving a metallic taste sensation and dry mouth. Visual disturbances including blurred vision, green vision and double vision.
Skin Problems Rash, hives. (urticaria), skin irritation, itching (pruritis), jaundice , Stevens-Johnson syndrome.
Other Fatigue, increased heart rate (tachycardia), hair loss, decreased red blood cell count (anemia), muscle pain (myalgia), joint pain (arthralagia).
References
- ^ Staff (2009). "Clinical Trials Update". Genetic Engineering & Biotechnology News 29 (8): 58
- ^ Callen JP, Hughes AP, Kulp-Shorten C (September 2001). "Subacute cutaneous lupus erythematosus induced or exacerbated by terbinafine: a report of 5 cases". Arch Dermatol 137 (9): 1196–8. doi:10.1001/archderm.137.9.1196. PMID 11559217. http://archderm.ama-assn.org/cgi/pmidlookup?view=long&pmid=11559217.
- ^ Reuters, US FDA approves oral granules for scalp ringworm
- ^ http://doublecheckmd.com/EffectsDetail.do?dname=Lamisil&sid=1510&eid=2237
- ^ http://www.mmm-online.com/australian-regulators-issue-warning-on-novartis-lamisil/article/104983/
- ^ http://www.healthline.com/goldcontent/terbinafine-1
Antifungals (D01 and J02) Wall/
membranetopical: Bifonazole, Clomidazole, Clotrimazole#, Croconazole, Econazole, Fenticonazole, Ketoconazole, Isoconazole, Miconazole#, Neticonazole, Oxiconazole, Sertaconazole, Sulconazole, Tioconazoletopical: (Fluconazole#, Fosfluconazole)
systemic: (Fluconazole, Hexaconazole, Itraconazole, Posaconazole, Voriconazole)topical: ThiabendazoleIntracellular Others Bromochlorosalicylanilide • Methylrosaniline • Tribromometacresol • Undecylenic acid • Polynoxylin • Chlorophetanol • Chlorphenesin • Ticlatone • Sulbentine • Ethyl hydroxybenzoate • Haloprogin • Salicylic acid • Selenium sulfide# • Ciclopirox • Amorolfine • Dimazole • Tolnaftate • Tolciclate • Sodium thiosulfate# • Whitfield's ointment# • Potassium iodide#
Tea tree oil • citronella oil • lemon grass • orange oil • patchouli • lemon myrtle
PCP: Pentamidine • Dapsone • Atovaquone#WHO-EM. ‡Withdrawn from market. Clinical trials: †Phase III. §Never to phase III Categories:- Antifungals
- Novartis
Wikimedia Foundation. 2010.
