- Allylamine
-
Allylamine 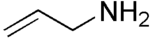 3-Amino-prop-1-eneOther names3-Aminopropene; 3-Aminopropylene; Monoallylamine; 2-Propenamine; 2-Propen-1-amine; Allyl amine
3-Amino-prop-1-eneOther names3-Aminopropene; 3-Aminopropylene; Monoallylamine; 2-Propenamine; 2-Propen-1-amine; Allyl amineIdentifiers CAS number 107-11-9 
ChemSpider 13835977 
UNII 48G762T011 
ChEMBL CHEMBL57286 
RTECS number BA5425000 Jmol-3D images Image 1 - C=CCN
Properties Molecular formula C3H7N Molar mass 57.09 g mol−1 Appearance Colorless liquid Density 0.7630 g/cm3, liquid Melting point -88 °C, 185 K, -126 °F
Boiling point 55-58 °C, 328-331 K, 131-136 °F
Acidity (pKa) 9.49[1] Hazards R-phrases R11 R23/24/25 R51/53 S-phrases S9 S16 S24/25 S45 S61 Main hazards Lachrymatory NFPA 704 Flash point -28 °C Related compounds Related amine Propylamine Related compounds Allyl alcohol  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Allylamine is an organic compound with the formula C3H5NH2. This colorless liquid is the simplest stable unsaturated amine.
Contents
Production
All three allylamines, mono-, di-, and triallylamine, are produced by the treating allyl chloride with ammonia followed by distillation.[2] Pure samples can be prepared by hydrolysis of allyl isothiocyanate.[3] It behaves as a typical amine.[4]
Reactions
Polymerization can be used to prepare the homopolymer (poly(allylamine)) or copolymers. The polymers are promising membranes for use in reverse osmosis.[2]
Safety
Allyl amine, like other allyl derivatives is a lacrymator and skin irritant. Its oral LD50 is 106 mg/kg for rats.
References
- ^ Hall, H.K., J. Am. Chem. Soc., 1957, 79, 5441.
- ^ a b Ludger Krähling, Jürgen Krey, Gerald Jakobson, Johann Grolig, Leopold Miksche "Allyl Compounds" in Ullmann's Encyclopedia of Industrial Chemistry, 2002, Wiley-VCH, Weinheim. doi: 10.1002/14356007.a01_425
- ^ M. T. Leffler (1943), "Allylamine", Org. Synth., http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=CV2P0024; Coll. Vol. 2: 24
- ^ Henk de Koning, W. Nico Speckamp "Allylamine" in Encyclopedia of Reagents for Organic Synthesis, 2001, John Wiley & Sons, Weinheim. doi:10.1002/047084289X.ra043 Article Online Posting Date: April 15, 2001
Categories:- Amines
- Alkenes
Wikimedia Foundation. 2010.

