- Dendritic spine
-
Dendritic spine 
Spiny dendrite of a striatal medium spiny neuron. 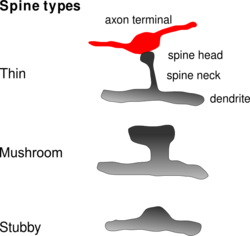
Common types of dendritic spines.(click to enlarge) Latin gemmula dendritica Code TH H2.00.06.1.00036 A dendritic spine (or spine) is a small membranous protrusion from a neuron's dendrite that typically receives input from a single synapse of an axon. Dendritic spines serve as a storage site for synaptic strength and help transmit electrical signals to the neuron's cell body. Most spines have a bulbous head (the spine head), and a thin neck that connects the head of the spine to the shaft of the dendrite. The dendrites of a single neuron can contain hundreds to thousands of spines. In addition to spines providing an anatomical substrate for memory storage and synaptic transmission, they may also serve to increase the number of possible contacts between neurons.
Contents
Distribution
Dendritic spines usually receive excitatory input from axons although sometimes both inhibitory and excitatory connections are made onto the same spine head. Spines are found on the dendrites of most principal neurons in the brain, including the pyramidal neurons of the neocortex, the medium spiny neurons of the striatum, and the Purkinje cells of the cerebellum.
Dendritic spines occur at a density of up to 50 spines/10 µm stretch of dendrite. Hippocampal and cortical pyramidal neurons may receive tens of thousands of mostly excitatory inputs from other neurons onto their equally numerous spines, whereas the number of spines on Purkinje neuron dendrites is an order of magnitude larger.
Morphology
Dendritic spines are small with spine head volumes ranging 0.01 µm3 to 0.8 µm3. Spines with strong synaptic contacts typically have a large spine head, which connect to the dendrite via a membranous neck. The most notable classes of spine shape are "thin", "stubby", "mushroom", and "branched". Electron microscopy studies have shown that there is a continuum of shapes between these categories. The variable spine shape and volume is thought to be correlated with the strength and maturity of each spine-synapse.
Biochemistry
Receptor activity
Dendritic spines express glutamate receptors (e.g. AMPA receptor and NMDA receptor) on their surface. The TrkB receptor for BDNF is also expressed on the spine surface, and is believed to play a role in spine survival. The tip of the spine contains an electrondense region referred to as the "postsynaptic density" (PSD). The PSD directly apposes the active zone of its synapsing axon and comprises ~10% of the spine's membrane surface area; neurotransmitters released from the active zone bind receptors in the postsynaptic density of the spine. One-half of the synapsing axons and dendritic spines are physically tethered by calcium-dependent cadherin, which forms cell-to-cell adherent junctions between two neurons.
Glutamate receptors (GluRs) are localized to the postsynaptic density, and are anchored by cytoskeletal elements to the membrane. They are positioned directly above their signaling machinery, which is typically tethered to the underside of the plasma membrane, allowing signals transmitted by the GluRs into the cytosol to be further propagated by their nearby signaling elements to activate signal transduction cascades. The localization of signaling elements to their GluRs is particularly important in ensuring signal cascade activation, as GluRs would be unable to affect particular downstream effects without nearby signalers.
Signaling from GluRs is mediated by the presence of an abundance of proteins, especially kinases, that are localized to the postsynaptic density. These include calcium-dependent calmodulin, CaMKII (calmodulin-dependent protein kinase II), PKC (Protein Kinase C), PKA (Protein Kinase A), Protein Phosphatase-1 (PP-1), and Fyn tyrosine kinase. Certain signalers, such as CaMKII, are upregulated in response to activity.
Spines are particularly advantageous to neurons by compartmentalizing biochemical signals. This can help to encode changes in the state of an individual synapse without necessarily affecting the state of other synapses of the same neuron. The length and width of the spine neck has a large effect on the degree of compartmentalization, with thin spines being the most biochemically isolated spines.
Cytoskeleton and Organelles
The cytoskeleton of dendritic spines is particularly important in their synaptic plasticity; without a dynamic cytoskeleton, spines would be unable to rapidly change their volumes or shapes in responses to stimuli. These changes in shape might affect the electrical properties of the spine. The cytoskeleton of dendritic spines is primarily made of filamentous actin (F-actin). While tubulin monomers and microtubule-associated proteins (MAPs) are present, organized microtubules are not present. Because spines have a cytoskeleton of primarily actin, this allows them to be highly dynamic in shape and size. The actin cytoskeleton directly determines the morphology of the spine, and actin regulators, small GTPases such as Rac, RhoA, and CDC42, rapidly modify this cytoskeleton. Overactive Rac1 results in consistently smaller dendritic spines.
In addition to their electrophysiological activity and their receptor-mediated activity, spines appear to be vesicularly active and may even translate proteins. Stacked discs of the smooth endoplasmic reticulum (SERs) have been identified in dendritic spines. Formation of this "spine apparatus" depends on the protein synaptopodin and is believed to play an important role in calcium handling. "Smooth" vesicles have also been identified in spines, supporting the vesicular activity in dendritic spines. The presence of polyribosomes in spines also suggests protein translational activity in the spine itself, not just in the dendrite.
Plasticity
See also: Synaptic plasticityAs aforementioned, dendritic spines are very "plastic", that is, spines change significantly in shape, volume, and number in small time courses. Because spines have a primarily actin cytoskeleton, they are dynamic, and the majority of spines change their shape within seconds to minutes because of the dynamicity of actin remodeling. Furthermore, spine number is very variable and spines come and go; in a matter of hours, 10-20% of spines can spontaneously appear or disappear on the pyramidal cells of the cerebral cortex, although the larger "mushroom"-shaped spines are the most stable.
Spine maintenance and plasticity is activity-dependent and activity-independent. BDNF partially determines spine levels[citation needed], and low levels of AMPA receptor activity is necessary to maintain spine survival, and synaptic activity involving NMDA receptors encourages spine growth. Furthermore, two-photon laser scanning microscopy and confocal microscopy have shown that spine volume changes depending on the types of stimuli that are presented to a synapse.
Importance to Learning and Memory
Evidence of Importance
Spine plasticity is implicated in motivation, learning, and memory. In particular, long-term memory is mediated in part by the growth of new dendritic spines (or the enlargement of pre-existing spines) to reinforce a particular neural pathway. Because dendritic spines are plastic structures whose lifespan is influenced by input activity, spine dynamics may play an important role in the maintenance of memory over a lifetime.
Age-dependent changes in the rate of spine turnover suggest that spine stability impacts developmental learning. In youth, dendritic spine turnover is relatively high and produces a net loss of spines, with the rate of the elimination of spines surpassing the rate of the formation of spines.[1][2][3] This high rate of spine turnover may characterize critical periods of development and reflect learning capacity in adolescence—different cortical areas exhibit differing levels of synaptic turnover during development, possibly reflecting varying critical periods for specific brain regions.[2][4] In adulthood, however, most spines remain persistent, and the half-life of spines increases.[1] This stabilization occurs due to a developmentally regulated slow-down of spine elimination, a process which may underlie the stabilization of memories in maturity.[1][2].
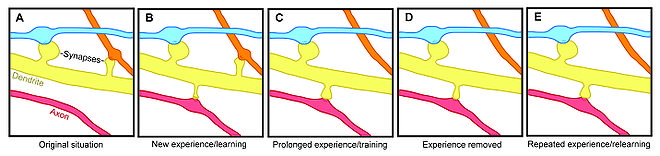
Experience-induced changes in dendritic spine stability also point to spine turnover as a mechanism involved in the maintenance of long-term memories, though it is unclear how sensory experience affects neural circuitry. Two general models might describe the impact of experience on structural plasticity. On the one hand, experience and activity may drive the discrete formation of relevant synaptic connections that store meaningful information in order to allow for learning. On the other hand, synaptic connections may be formed in excess, and experience and activity may lead to the pruning of extraneous synaptic connections.[1]
In lab animals of all ages, environmental enrichment has been related to dendritic branching, spine density, and overall number of synapses.[1] In addition, skill training has been shown to lead to the formation and stabilization of new spines while destabilizing old spines,[5][6] suggesting that the learning of a new skill involves a rewiring process of neural circuits. Since the extent of spine remodeling correlates with success of learning, this suggests a crucial role of synaptic structural plasticity in memory formation.[5] In addition, changes in spine stability and strengthening occur rapidly and have been observed within hours after training.[4][6]
Conversely, while enrichment and training are related to increases in spine formation and stability, long-term sensory deprivation leads to a decrease in the rate of spine elimination[1][2] and therefore impacts long-term neural circuitry. Upon restoring sensory experience after deprivation in adolescence, spine elimination is accelerated, suggesting that experience plays an important role in the net loss of spines during development.[2] In addition, other sensory deprivation paradigms—such as whisker trimming—have been shown to increase the stability of new spines.[7]
Research in neurological diseases and injuries shed further light on the nature and importance of spine turnover. After stroke, a marked increase in structural plasticity occurs near the trauma site, and a five- to eightfold increase from control rates in spine turnover has been observed.[8] Dendrites disintegrate and reassemble rapidly during ischemia—as with stroke, survivors showed an increase in dendritic spine turnover.[9] While a net loss of spines is observed in Alzheimer's disease and cases of mental retardation, cocaine and amphetamine use have been linked to increases in dendritic branching and spine density in the prefrontal cortex and the nucleus accumbens.[10] Because significant changes in spine density occur in various brain diseases, this suggests a balanced state of spine dynamics in normal circumstances, which may be susceptible to disequilibrium under varying pathological conditions.[10]
There is also some evidence for loss of dendritic spines as a consequence of aging. One study using mice has noted a correlation between age-related reductions in spine densities in the hippocampus that and age-dependent declines in hippocampal learning and memory.[11]
Importance Contested
Despite experimental findings that suggest a role for dendritic spine dynamics in mediating learning and memory, the degree of structural plasticity’s importance remains debatable. For instance, studies estimate that only a small portion of spines formed during training actually contribute to lifelong learning.[5] In addition, the formation of new spines may not significantly contribute to the connectivity of the brain, and spine formation may not bear as much of an influence on memory retention as other properties of structural plasticity, such as the increase in size of spine heads.[12]
Electrotonic properties
Electrotonic conduction refers to the passive conduction of current. Dendritic spines have a number of specific electrotonic properties. A dendritic spine has high input resistance, the resistance increases with smallness of headsize and narrowness of stemsize. The capacitance of the membranes of spines is relatively small with the result that synaptic potentials can be relatively fast. The capacitance of the whole dendrite however becomes higher as the number of spines increases. Because there is an impedance mismatch between the dendritic spine and the dendrite, it is necessary with active signal boosting. The impedance mismatch also causes the spine to follow the potential of the parent dendrite.
Modelling
Theoreticians have for decades hypothesized about the potential electrical function of spines, yet our inability to examine their electrical properties has until recently stopped theoretical work from progressing too far. Recent advances in imaging techniques along with increased use of two-photon glutamate uncaging have led to a wealth of new discoveries; we now suspect that there are voltage-dependent sodium,[13] potassium,[14] and calcium[15] channels in the spine heads.
Cable theory provides the theoretical framework behind the most "simple" method for modelling the flow of electrical currents along passive neural fibres. Each spine can be treated as two compartments, one representing the neck, the other representing the spine head. The compartment representing the spine head alone should carry the active properties.
Baer and Rinzel's Continuum Model
To facilitate the analysis of interactions between many spines, Baer & Rinzel formulated a new cable theory for which the distribution of spines is treated as a continuum.[16] In this representation, spine head voltage is the local spatial average of membrane potential in adjacent spines. The formulation maintains the feature that there is no direct electrical coupling between neighboring spines; voltage spread along dendrites is the only way for spines to interact.
The Spike-Diffuse-Spike Model
The SDS model was intended as a computationally simple version of the full Baer and Rinzel model.[17] It was designed to be analytically tractable and have as few free parameters as possible while retaining those of greatest significance, such as spine neck resistance. The model drops the continuum approximation and instead uses a passive dendrite coupled to excitable spines at discrete points. Membrane dynamics in the spines are modelled using integrate and fire processes. The spike events are modelled in a discrete fashion with the wave form conventionally represented as a rectangular function.
Modelling spine calcium transients
Calcium transients in spines are a key trigger for synaptic plasticity.[18] NMDA receptors, which have a high permeability for calcium, only conduct ions if the membrane potential is suffiently depolarized. The amount of calcium entering a spine during synaptic activity therefore depends on the depolarization of the spine head. Evidence from calcium imaging experiments (two-photon microscopy) and from compartmental modelling indicates that spines with high resistance necks experience larger calcium transients during synaptic activity.[19]
Development
Dendritic spines are believed to develop from filopodia. During synaptogenesis, dendrites rapidly sprout and retract filopodia, small membrane organelle-lacking membranous protrusions. During the first week of birth, the brain is predominated by filopodia, which eventually develop synapses. However, after this first week, filopodia are replaced by spiny dendrites but also small, stubby spines that protrude from spiny dendrites. In the development of certain filopodia into spines, filopodia recruit presynaptic contact to the dendrite, which encourages the production of spines to handle specialized postsynaptic contact with the presynaptic protrusions.
Spines, however, require maturation after formation. Immature spines have impaired signaling capabilities, and typically lack "heads" (or have very small heads), only necks, while matured spines maintain both heads and necks.
Pathology
Cognitive disorders such as ADHD, autism, mental retardation, and fragile X syndrome, may be resultant from abnormalities in dendritic spines, especially the number of spines and their maturity. The ratio of matured to immature spines is important in their signaling, as immature spines have impaired synaptic signaling. Fragile X syndrome is characterized by an overabundance of immature spines that have multiple filopodia in cortical dendrites.
References
- ^ a b c d e f Alvarez, V.; Sabatini, B. (2007). "Anatomical and Physiological Plasticity of Dendritic Spines". Annual Review of Neuroscience 30: 79–97. doi:10.1146/annurev.neuro.30.051606.094222. PMID 17280523.
- ^ a b c d e Zuo, A.; Lin; Chang, P.; Gan, W. B. (2005). "Development of long-term dendritic spine stability in diverse regions of cerebral cortex". Neuron 46 (2): 181–189. doi:10.1016/j.neuron.2005.04.001. PMID 15848798.
- ^ Holtmaat, A. J.; Trachtenberg, J. T.; Wilbrecht, L.; Shepherd, G. M.; Zhang, X.; et al. (2005). "Transient and persistent dendritic spines in the neocortex in vivo". Neuron 45 (2): 279–291. doi:10.1016/j.neuron.2005.01.003. PMID 15664179.
- ^ a b Roberts, T.; Tschida, K.; Klein, M.; Mooney, R. (2010). "Rapid spine stabilization and synaptic enhancement at the onset of behavioural learning". Nature 463 (7283): 948–952. doi:10.1038/nature08759. PMC 2918377. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2918377.
- ^ a b c Yang, G.; Pan, F.; Gan, W. B. (2009). "Stably maintained dendritic spines are associated with lifelong memories". Nature 462 (7275): 920–924. doi:10.1038/nature08577. PMID 19946265.
- ^ a b Xu, T.; Yu, X.; Perlik, A.; Tobin, W.; Zweig, J.; Tennant, K.; Jones, T.; Zuo, Y. (2009). "Rapid formation and selective stabilization of synapses for enduring motor memories". Nature 462 (7275): 915–919. doi:10.1038/nature08389. PMC 2844762. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2844762.
- ^ Holtmaat, A.; Wilbrecht, L.; Knott, G. W.; Welker, E.; Svoboda, K. (2006). "Experience-dependent and cell-type-specific spine growth in the neocortex". Nature 441 (7096): 979–983. doi:10.1038/nature04783. PMID 16791195.
- ^ Brown, C.; Li, P.; Boyd, J.; Delaney, K.; Murphy, T. (2007). "Extensive Turnover of Dendritic Spines and Vascular Remodeling in Cortical Tissues Recovering from Stroke". Journal of Neuroscience 27 (15): 4101–4109. doi:10.1523/JNEUROSCI.4295-06.2007. PMID 17428988.
- ^ Brown, C.; Murphy, T. H. (2008). "Livin' on the edge: imaging dendritic spine turnover in the peri-infarct zone during ischemic stroke and recovery". Neuroscientist 14 (2): 139–146. doi:10.1177/1073858407309854. PMID 18039977.
- ^ a b Bhatt, D.; Zhang, S.; Gan, W. B. (2009). Dendritic Spine Dynamics. 71. 261–282. doi:10.1146/annurev.physiol.010908.163140. PMID 19575680.
- ^ von Bohlen und Halbach O, Zacher C, Gass P, Unsicker K (2006). "Age-related alterations in hippocampal spines and deficiencies in spatial memory in mice". J Neurosci Res 83: 525–531. PMID 16447268.
- ^ Harris, K.; Fiala, J.; Ostroff, L. (2003). "Structural Changes at Dendritic Spine Synapses during Long-Term Potentiation". Philosophical Transactions: Biological Sciences 358 (1432): 745–748. doi:10.1098/rstb.2002.1254. PMC 1693146. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1693146.
- ^ Araya, R.; Nikolenko, V.; Eisenthal, K. B.; Yuste, R. (2007). "Sodium channels amplify spine potentials". PNAS 104 (30): 12347–12352. doi:10.1073/pnas.0705282104. PMC 1924793. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1924793.
- ^ Ngo-Anh, T. J.; Bloodgood, B. L.; Lin, M.; Sabatini, B. L.; Maylie, J.; Adelman, J. P. (2005). "SK channels and NMDA receptors form a Ca2+-mediated feedback loop in dendritic spines". Nature Neuroscience 8 (5): 642–649. doi:10.1038/nn1449. PMID 15852011.
- ^ Yuste, R.; Denk, W. (1995). "Dendritic spines as basic functional units of neuronal integration". Nature 375 (6533): 682–684. doi:10.1038/375682a0. PMID 7791901.
- ^ Baer, S. M.; Rinzel, J. (1991). "Propagation of dendritic spikes mediated by excitable spines: a continuum theory". Journal of Neurophysiology 65 (4): 874–890. PMID 2051208.
- ^ Coombes, S.; Bressloff, P. C. (2000). "Solitary Waves in a Model of Dendritic Cable with Active Spines". SIAM Journal on Applied Mathematics 61 (2): 432–453. JSTOR 3061734.
- ^ Nevian, T.; Sakmann, B. (2006). "Spine Ca2+ signaling in spike-timing-dependent plasticity". Journal of Neuroscience 26 (43): 11001–11013. doi:10.1523/JNEUROSCI.1749-06.2006. PMID 17065442.
- ^ Grunditz, A.; Holbro, N.; Tian, L.; Zuo, Y.; Oertner, T. G. (2008). "Spine neck plasticity controls postsynaptic calcium signals through electrical compartmentalization". Journal of Neuroscience 28 (50): 13457–13466. doi:10.1523/JNEUROSCI.2702-08.2008. PMID 19074019.
Further reading
- Sudhof, T. C.; Stevens, C. F.; Cowan, W. M. (2001). Synapses. Baltimore: The Johns Hopkins University Press. ISBN 0801864984.
- Levitan, I. B.; Kaczmarek, L. K. (2002). The Neuron: Cell and Molecular Biology (Third ed.). New York: Oxford University Press. ISBN 0195145224.
- Plummer, M.; Page, C.; Firestein, B.; Davis, R. (?[Full citation needed]). Advanced Neurobiology I/Neuroscience Lecture Notes. unpublished.
- Nimchinsky E, Sabatini B, Svoboda K (2002). "Structure and function of dendritic spines". Annu Rev Physiol 64: 313–53. doi:10.1146/annurev.physiol.64.081501.160008. PMID 11826272.
- Matsuzaki M, Honkura N, Ellis-Davies G, Kasai H (2004). "Structural basis of long-term potentiation in single dendritic spines". Nature 429 (6993): 761–6. doi:10.1038/nature02617. PMID 15190253.
- Yuste R, Majewska A, Holthoff K (2000). "From form to function: calcium compartmentalization in dendritic spines". Nat Neurosci 3 (7): 653–9. doi:10.1038/76609. PMID 10862697.
- Lieshoff C, Bischof H (2003). "The dynamics of spine density changes". Behav Brain Res 140 (1–2): 87–95. doi:10.1016/S0166-4328(02)00271-1. PMID 12644282.
- Kasai H, Matsuzaki M, Noguchi J, Yasumatsu N (2002). "Dendritic spine structures and functions". Nihon Shinkei Seishin Yakurigaku Zasshi 22 (5): 159–64. PMID 12451686.
- Lynch G, Rex CS, Gall CM (2007). "LTP consolidation: substrates, explanatory power, and functional significance". Neuropharmacology 52 (1): 12–23. doi:10.1016/j.neuropharm.2006.07.027. PMID 16949110.
External links
- The Manual of Cellular and Molecular Function - Dendritic spines
- Spiny Dendrite - Cell Centered Database
Histology: nervous tissue (TA A14, GA 9.849, TH H2.00.06, H3.11) CNS GeneralGrey matter · White matter (Projection fibers · Association fiber · Commissural fiber · Lemniscus · Funiculus · Fasciculus · Decussation · Commissure) · meningesOtherPNS GeneralPosterior (Root, Ganglion, Ramus) · Anterior (Root, Ramus) · rami communicantes (Gray, White) · Autonomic ganglion (Preganglionic nerve fibers · Postganglionic nerve fibers)Myelination: Schwann cell (Neurolemma, Myelin incisure, Myelin sheath gap, Internodal segment)
Satellite glial cellNeurons/
nerve fibersPartsPerikaryon (Axon hillock)
Axon (Axon terminals, Axoplasm, Axolemma, Neurofibril/neurofilament)
Dendrite (Nissl body, Dendritic spine, Apical dendrite/Basal dendrite)TypesGSA · GVA · SSA · SVA
fibers (Ia, Ib or Golgi, II or Aβ, III or Aδ or fast pain, IV or C or slow pain)GSE · GVE · SVE
Upper motor neuron · Lower motor neuron (α motorneuron, γ motorneuron, β motorneuron)Termination SynapseCategories:
Wikimedia Foundation. 2010.