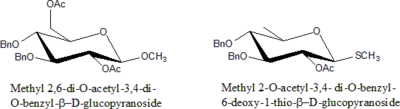- Monosaccharide nomenclature
-
Monosaccharide nomenclature is a set of conventions used in chemistry to name the compounds known as monosaccharides or "simple sugars" — the basic structural units of carbohydrates, which cannot be hydrolysed into simpler units.[1]
Contents
Systematic name of molecular graph
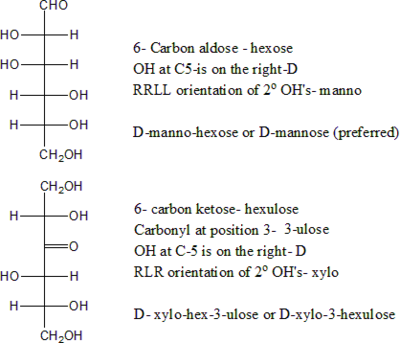
The elementary formula of a simple monosaccharide is CnH2nOn, where the integer n is at least 3 and rarely greater than 7. Simple monosaccharides may be named generically according on the number of carbon atoms n: trioses, tetroses, pentoses, hexoses, etc.
Every simple monosaccharide has an acyclic (open chain) form, which can be written as H-(CH(OH))x-(C=O)-(CH(OH))y-H; that is, a straight chain of carbon atoms, one of which is a carbonyl group, all the others bearing an hydrogen -H and a hydroxyl -OH each, with one extra hydrogen at either end. The carbons of the chain are conventionally numbered from 1 to n, starting from the end which is closest to the carbonyl.
If the carbonyl is at the very beginning of the chain (carbon 1), the monosaccharide is said to be an aldose, otherwise it is a ketose. These names can be combined with the chain length prefix, as in aldohexose or ketopentose. Most ketoses found in nature have the carbonyl in position 2; when that is not the case, one uses a numeric prefix to indicate the carbonyl's position. Thus for example, aldohexose means H(C=O)(CHOH)5H, ketopentose means H(CHOH)(C=O)(CHOH)3H, and 3-ketopentose means H(CHOH)2(C=O)(CHOH)2H.
An alternative nomenclature uses the suffix '-ose' only for aldoses, and '-ulose' for ketoses. The position of the carbonyl (when it is not 1 or 2) is indicated by a numerical infix. For example, hexose in this nomenclature means H(C=O)(CHOH)5H, pentulose means H(CHOH)(C=O)(CHOH)3H, and hexa-3-ulose means H(CHOH)2(C=O)(CHOH)3H.
Naming of acyclic stereoisomers
Open-chain monosaccharides with same molecular graph may exist as two or more stereoisomers. The Fischer projection is a systematic way of drawing the skeletal formula of an open-chain monosaccharide so that each stereoisomer is uniquely identified.
Two isomers whose molecules are mirror-images of each other are identified by prefixes 'D-' or 'L-', according to the handedness of the chiral carbon atom that is farthest from the carbonyl. In the Fischer projection, that is the second carbon from the bottom; the prefix is 'D-' or 'L-' according to whether the hydroxyl on that carbon lies to the right or left of the backbone, respectively.
If the molecular graph is symmetrical (H(CHOH)x(CO)(CHOH)xH) and the two halves are mirror images of each other, then the molecule is identical to its mirror image, and there is no 'L-' form.
A distinct common name, such as "glucose" or "ribose", is traditionally assigned to each pair of mirror-image stereoisomers, and to each achiral stereoisomer. These names have standard three-letter abbreviations, such as 'Glu' for glucose and 'Rib' for ribose.
Another nomenclature uses the systematic name of the molecular graph, a 'D-' or 'L-' prefix to indicate the position of the last chiral hydroxyl on the Fischer diagram (as above), and another italic prefix to indicate the positions of the remaining hydroxyls relative to the first one, read from bottom to top in the diagram, skipping the keto group if any. These prefixes are attached to the systematic name of the molecular graph. So for example, D-glucose is D-gluco-hexose, D-ribose is D-ribo-pentose, and D-psicose is D-ribo-hexulose. Note that, in this nomenclature, mirror-image isomers differ only in the 'D'/'L' prefix, even though all their hydroxyls are reversed.
The following tables shows the Fischer projections of selected monosaccharides (in open-chain form), with their conventional names. The table shows all aldoses with 3 to 6 carbon atoms, and a few ketoses. For chiral molecules, only the 'D-' form (with the next-to-last hydroxyl on the right side) is shown; the corresponding forms have mirror-image structures. Some of these monosaccharides are only synthetically prepared in the laboratory and not found in nature.
Names of aldoses
Aldotrioses
Trioses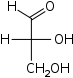
D-Glyceraldehyde
Aldotetroses
Tetroses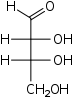
D-Erythrose
erythro-
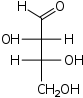
D-Threose
threo-
Aldopentoses
Pentoses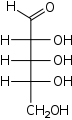
D-Ribose
ribo-
Rib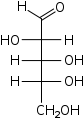
D-Arabinose
arabino-
Ara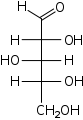
D-Xylose
xylo-
Xyl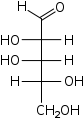
D-Lyxose
lyxo-
LyxAldohexoses
Hexoses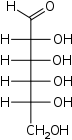
D-Allose
allo-
Ala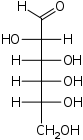
D-Altrose
altro-
Alt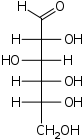
D-Glucose
gluco-
Glc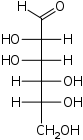
D-Mannose
manno-
Man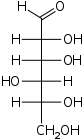
D-Gulose
gulo-
Gul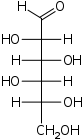
D-Idose
ido-
Ido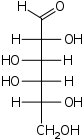
D-Galactose
galacto-
Gal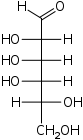
D-Talose
talo-
TalNames of ketoses
Ketotrioses
Triuloses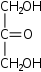
Glycerone
Ketotetrose
Tetruloses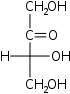
D-Erythrulose
glycero-
Ketopentoses
Pentuloses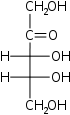
D-Ribulose
erythro-
Rul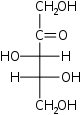
D-Xylulose
threo-
XulKetohexoses
Hexuloses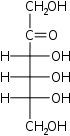
D-Psicose
ribo-
Psi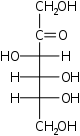
D-Fructose
arabino-
Fru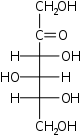
D-Sorbose
xylo-
Sor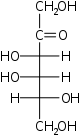
D-Tagatose
lyxo-
TagNames of 3-ketoses
3-Ketopentoses
Penta-3-uloses
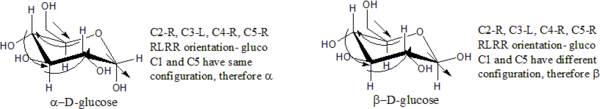
edit] Cyclic formsFor monosaccharides in their cyclic form, an infix is placed before the '-ose', '-ulose', or 'n-ulose' suffix to specify the ring size. The infix is "furan" for a 5-atom ring, "pyran" for 6, "septan" for 7, and so on).
Ring closure creates another chiral center at the anomeric carbon (the one with the hemiacetal or acetal functionality), and therefore each open-chain stereoisomer gives rise to two distinct stereoisomers (anomers). These are identified by the prefixes 'α-' and 'β-', according to he configuration of the anomeric carbon relative to that of furthest stereocenter along the open chain. To determine if the sugar is α or β, the structure is drawn in a Fischer projection; if the endocyclic oxygen (O5) and exocyclic oxygen (O1) are cis, the sugar is α; if they are trans, the sugar is β.[2][3]


Examples
Glycosides
Glycosides are saccharides in which the hydroxyl -OH at the anomeric centre is replaced by an oxygen-bridged group -OR. The carbohydrate part of the molecule is called glycone, the -O- bridge is the glycosisdic oxygen, and the attached group is the aglycone. Glycosides are named by giving the aglyconic alcohol HOR, followed by the saccahride name with the '-e' ending repalced by '-ide'; as in [[phenol D-glucopyrnoside]].


Modified Sugars
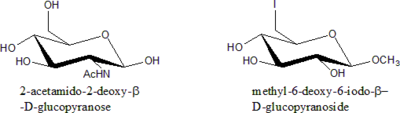
Deoxy sugars
Modification of sugar is generally done by replacing one or more –OH group with other functional groups at all position except C-1. Since all these cases involves the removal of an –OH group, they are all deoxy sugars.
Rules for Nomenclature of Modified Sugars:
- State the Sugar is deoxy sugar.
- Specify the position of deoxygenation.
- If there is a substituent other than H in the place of –OH, specify what it is.
- Specify the relative configuration of all stereogenic centres (manno, gluco etc.).
- Specify the ring size (furanose, pyranose etc.) and anomeric configuration ( a or b).
- State the chain length only in situation where –OH is replaced with H.
- Alphabetize all the substituent groups (deoxy, -iodo, -amino etc.). Di-, tri- etc. prefixes do not count.
Examples
Protected Sugars
Sugars in which –OH is protected by some modification are called protected sugars.
Rules for Nomenclature for Protected Sugars:
- Specify the number of particular protecting groups (di, tri, tetra etc.).
- List groups alphabetically along with all other substituents ( di, tri prefixes do not count).
See also
- Carbohydrate conformation
- Polysaccharide
- Oligosaccharide
- Oligosaccharide nomenclature
References
- ^ Carbohydrate Nomenclature Archived 22 January 2011 at WebCite
- ^ IUPAC Sec 2-Carb6.2 Archived 22 January 2011 at WebCite
- ^ Trivial Nomenclature of Monosaccharides Archived 22 January 2011 at WebCite
Types of carbohydrates General: Geometry Monosaccharides Aldodiose (Glycolaldehyde)Ketopentose (Ribulose, Xylulose)
Aldopentose (Ribose, Arabinose, Xylose, Lyxose)
Deoxy sugar (Deoxyribose)Ketoheptose (Sedoheptulose, Mannoheptulose)>7Multiple Other oligosaccharidesGlucose/Glucan: Glycogen · Starch (Amylose, Amylopectin) · Cellulose · Dextrin/Dextran · Beta-glucan (Zymosan, Lentinan, Sizofiran) · Maltodextrin
Fructose/Fructan: Inulin · Levan beta 2→6
N-Acetylglucosamine: Chitinbiochemical families: prot · nucl · carb (glpr, alco, glys) · lipd (fata/i, phld, strd, gllp, eico) · amac/i · ncbs/i · ttpy/i Categories:- Organic chemistry
- Carbohydrates
- Carbohydrate chemistry
Wikimedia Foundation. 2010.
Look at other dictionaries:
Monosaccharide — Monosaccharides (from Greek monos: single, sacchar: sugar) are the most basic units of biologically important carbohydrates. They are the simplest form of sugar and are usually colorless, water soluble, crystalline solids. Some monosaccharides… … Wikipedia
Monosaccharide — Ose Pour les articles homonymes, voir Ose (homonymie). Les oses (ou monosaccharides) sont les monomères des glucides. Ils ne sont pas hydrolysables. Tout comme les diholosides (ou disaccharides), ils possèdent un pouvoir sucrant, et sont solubles … Wikipédia en Français
Oligosaccharide nomenclature — Oligosaccharides and polysaccharides are an important class of polymeric carbohydrates found in virtually all living entities.[1] Their structural features make their nomenclature challenging and their roles in living systems make their… … Wikipedia
carbohydrate — /kahr boh huy drayt, beuh /, n. any of a class of organic compounds that are polyhydroxy aldehydes or polyhydroxy ketones, or change to such substances on simple chemical transformations, as hydrolysis, oxidation, or reduction, and that form the… … Universalium
Carbohydrate — Carbohydrates (from hydrates of carbon ) or saccharides (Greek σάκχαρον meaning sugar ) are the most abundant of the four major classes of biomolecules, which also include proteins, lipids and nucleic acids. They fill numerous roles in living… … Wikipedia
Congenital disorder of glycosylation — Congenital disorders of glycosylation Classification and external resources ICD 10 E77.8 ICD 9 271.8 … Wikipedia
Glucose — This article is about the naturally occurring D form of glucose. For the L form, see L Glucose. D glucose … Wikipedia
heterocyclic compound — Any of a class of organic compounds whose molecules contain one or more rings of atoms with at least one atom (the heteroatom) being an element other than carbon, most frequently oxygen, nitrogen, or sulfur. As in regular cyclic hydrocarbons,… … Universalium
Disaccharide — A disaccharide or biose[1] is the carbohydrate formed when two monosaccharides undergo a condensation reaction which involves the elimination of a small molecule, such as water, from the functional groups only. Like monosaccharides, disaccharides … Wikipedia
Polyphenol — Plant derived polyphenol, tannic acid, formed by esterification of ten equivalents of the phenylpropanoid derived gallic acid to a monosaccharide (glucose) core from primary metabolism … Wikipedia