- Deoxygenation
-
Deoxygenation is a chemical reaction involving the removal of molecular oxygen (O2) from a reaction mixture or solvent, or the removal of oxygen atoms from a molecule.
Classic representatives of deoxygenation are:
- the replacement of a hydroxyl group by hydrogen (A-OH → A-H) in the Barton-McCombie deoxygenation or in the Markó-Lam deoxygenation
- the replacement of an oxo group by two hydrogen atoms (A=O → A) in the Wolff-Kishner reduction
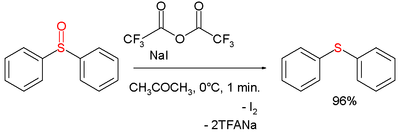
A chemical reagent for the deoxygenation of many sulfur and nitrogen oxo compounds is the trifluoroacetic anhydride / sodium iodide combination [1] for example in the deoxygenation of the sulfoxide diphenylsulfoxide to the sulfide diphenylsulfide:
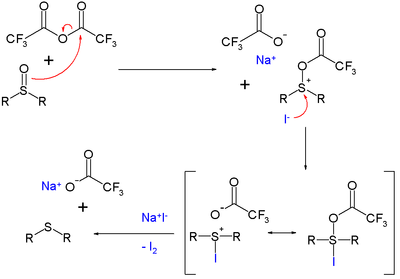
The reaction mechanism is based on activation of the sulfoxide by a trifluoroacetyl group and oxidation of iodine. Iodine is formed quantitatively in this reaction and therefore the reagent is used for the analytical detection of many oxo compounds.
See also
- Preparation of stable carbenes
- Hydrogen deoxygenation purifier
References
Categories:- Organic redox reactions
Wikimedia Foundation. 2010.