- MicroRNA
-
A microRNA (abbreviated miRNA) is a short ribonucleic acid (RNA) molecule found in eukaryotic cells. A microRNA molecule has very few nucleotides (an average of 22) compared with other RNAs.
miRNAs are post-transcriptional regulators that bind to complementary sequences on target messenger RNA transcripts (mRNAs), usually resulting in translational repression or target degradation and gene silencing.[1][2] The human genome may encode over 1000 miRNAs,[3][4] which may target about 60% of mammalian genes[5][6] and are abundant in many human cell types.[7]
miRNAs show very different characteristics between plants and metazoans. In plants the miRNA complementarity to its mRNA target is nearly perfect, with no or few mismatched bases. In metazoans, on the other hand, miRNA complementarity typically encompasses the 5' bases 2-7 of the microRNA, the microRNA seed region,[5][8] and one miRNA can target many different sites on the same mRNA or on many different mRNAs. Another difference is the location of target sites on mRNAs. In metazoans, the miRNA target sites are in the three prime untranslated regions (3'UTR) of the mRNA. In plants, targets can be located in the 3' UTR but are more often in the coding region itself.[9] MiRNAs are well conserved in eukaryotic organisms and are thought to be a vital and evolutionarily ancient component of genetic regulation.[10][11][12][13]
The first miRNAs were characterized in the early 1990s. However, miRNAs were not recognized as a distinct class of biological regulators with conserved functions until the early 2000s. Since then, miRNA research has revealed multiple roles in negative regulation (transcript degradation and sequestering, translational suppression) and possible involvement in positive regulation (transcriptional and translational activation). By affecting gene regulation, miRNAs are likely to be involved in most biological processes.[14][15][16][17][18][19][20] Different sets of expressed miRNAs are found in different cell types and tissues.[21]
Aberrant expression of miRNAs has been implicated in numerous disease states, and miRNA-based therapies are under investigation.[22][23][24]
Contents
History
MicroRNAs were discovered in 1993 by Victor Ambros, Rosalind Lee and Rhonda Feinbaum during a study of the gene lin-14 in C. elegans development.[25] They found that LIN-14 protein abundance was regulated by a short RNA product encoded by the lin-4 gene. A 61-nucleotide precursor from the lin-4 gene matured to a 22-nucleotide RNA that contained sequences partially complementary to multiple sequences in the 3’ UTR of the lin-14 mRNA. This complementarity was both necessary and sufficient to inhibit the translation of the lin-14 mRNA into the LIN-14 protein. Retrospectively, the lin-4 small RNA was the first microRNA to be identified, though at the time, it was thought to be a nematode idiosyncrasy. Only in 2000 was a second RNA characterized: let-7, which repressed lin-41, lin-14, lin-28, lin-42, and daf-12 expression during developmental stage transitions in C. elegans. let-7 was soon found to be conserved in many species,[26][27] indicating the existence of a wider phenomenon.
Nomenclature
Under a standard nomenclature system, names are assigned to experimentally confirmed miRNAs before publication of their discovery.[28][29] The prefix "mir" is followed by a dash and a number, the latter often indicating order of naming. For example, mir-123 was named and likely discovered prior to mir-456. The uncapitalized "mir-" refers to the pre-miRNA, while a capitalized "miR-" refers to the mature form. miRNAs with nearly identical sequences bar one or two nucleotides are annotated with an additional lower case letter. For example, miR-123a would be closely related to miR-123b. Pre-miRNAs that lead to 100% identical mature miRNAs but that are located at different places in the genome are indicated with an additional dash-number suffix. For example, the pre-miRNAs hsa-mir-194-1 and hsa-mir-194-2 lead to an identical mature miRNA (hsa-miR-194) but are located in different regions of the genome. Species of origin is designated with a three-letter prefix, e.g., hsa-miR-123 is a human (Homo sapiens)miRNA and oar-miR-123 is a sheep (Ovis aries) miRNA. Other common prefixes include 'v' for viral (miRNA encoded by a viral genome) and 'd' for Drosophila miRNA (a fruit fly commonly studied in genetic research). When two mature microRNAs originate from opposite arms of the same pre-miRNA, they are denoted with a -3p or -5p suffix. (In the past, this distinction was also made with 's' (sense) and 'as' (antisense)). When relative expression levels are known, an asterisk following the name indicates an miRNA expressed at low levels relative to the miRNA in the opposite arm of a hairpin. For example, miR-123 and miR-123* would share a pre-miRNA hairpin, but more miR-123 would be found in the cell.
Biogenesis
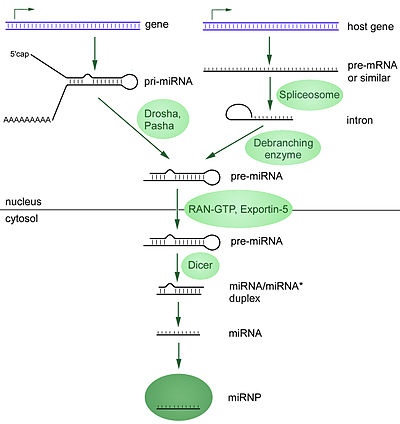 MicroRNAs are produced from either their own genes or from introns. A video of this process can be found here.
MicroRNAs are produced from either their own genes or from introns. A video of this process can be found here.
The majority of the characterized miRNA genes are intergenic or oriented antisense to neighboring genes and are therefore suspected to be transcribed as independent units[30] [30][31][32][33] As much as 40% of miRNA genes may lie in the introns of protein and non-protein coding genes or even in exons of long nonprotein-coding transcripts.[34] These are usually, though not exclusively, found in a sense orientation.[35][36] and thus usually are regulated together with their host genes.[34][37][38] Other miRNA genes showing a common promoter include the 42-48% of all miRNAs originating from polycistronic units containing multiple discrete loops from which mature miRNAs are processed,[31][39] although this does not necessarily mean the mature miRNAs of a family will be homologous in structure and function. The promoters mentioned have been shown to have some similarities in their motifs to promoters of other genes transcribed by RNA polymerase II such as protein coding genes.[31][40] The DNA template is not the final word on mature miRNA production: 6% of human miRNAs show RNA editing ( IsomiRs), the site-specific modification of RNA sequences to yield products different from those encoded by their DNA. This increases the diversity and scope of miRNA action beyond that implicated from the genome alone.
Transcription
miRNA genes are usually transcribed by RNA polymerase II (Pol II).[31][40] The polymerase often binds to a promoter found near the DNA sequence encoding what will become the hairpin loop of the pre-miRNA. The resulting transcript is capped with a specially-modified nucleotide at the 5’ end, polyadenylated with multiple adenosines (a poly(A) tail),[31][35] and spliced. Animal miRNAs are initially transcribed as part of one arm of an ∼80 nucleotide RNA stem-loop that in turn forms part of a several hundred nucleotides long miRNA precursor termed a primary miRNA (pri-miRNA)s.[31][35] When a stem-loop precursor is found in the 3’ UTR, a transcript may serve as a pri-miRNA and a mRNA.[35] RNA polymerase III (Pol III) transcribes some miRNAs, especially those with upstream Alu sequences, transfer RNAs (tRNAs), and mammalian wide interspersed repeat (MWIR) promoter units.[41]
Nuclear processing
A single pri-miRNA may contain from one to six miRNA precursors. These hairpin loop structures are composed of about 70 nucleotides each. Each hairpin is flanked by sequences necessary for efficient processing. The double-stranded RNA structure of the hairpins in a pri-miRNA is recognized by a nuclear protein known as DiGeorge Syndrome Critical Region 8 (DGCR8 or "Pasha" in invertebrates), named for its association with DiGeorge Syndrome. DGCR8 associates with the enzyme Drosha, a protein that cuts RNA, to form the "Microprocessor" complex.[42] In this complex, DGCR8 orients the catalytic RNase III domain of Drosha to liberate hairpins from pri-miRNAs by cleaving RNA about eleven nucleotides from the hairpin base (two helical RNA turns into the stem). The product resulting has a two-nucleotide overhang at its 3’ end; it has 3' hydroxyl and 5' phosphate groups. It is often termed as a pre-miRNA (precursor-miRNA).
pre-miRNAs that are spliced directly out of introns, bypassing the Microprocessor complex, are known as "Mirtrons." Originally thought to exist only in Drosophila and C. elegans, mirtrons have now been found in mammals.[43]
Perhaps as many as 16% of pri-miRNAs may be altered through nuclear RNA editing.[44][45][46] Most commonly, enzymes known as adenosine deaminases acting on RNA (ADARs) catalyze adenosine to inosine (A to I) transitions. RNA editing can halt nuclear processing (for example, of pri-miR-142, leading to degradation by the ribonuclease Tudor-SN) and alter downstream processes including cytoplasmic miRNA processing and target specificity (e.g., by changing the seed region of miR-376 in the central nervous system).[44]
Nuclear export
pre-miRNA hairpins are exported from the nucleus in a process involving the nucleocytoplasmic shuttle Exportin-5. This protein, a member of the karyopherin family, recognizes a two-nucleotide overhang left by the RNase III enzyme Drosha at the 3' end of the pre-miRNA hairpin. Exportin-5-mediated transport to the cytoplasm is energy-dependent, using GTP bound to the Ran protein.[47]
Cytoplasmic processing
In cytoplasm, the pre-miRNA hairpin is cleaved by the RNase III enzyme Dicer.[48] This endoribonuclease interacts with the 3' end of the hairpin and cuts away the loop joining the 3' and 5' arms, yielding an imperfect miRNA:miRNA* duplex about 22 nucleotides in length.[48] Overall hairpin length and loop size influence the efficiency of Dicer processing, and the imperfect nature of the miRNA:miRNA* pairing also affects cleavage.[48][49] Although either strand of the duplex may potentially act as a functional miRNA, only one strand is usually incorporated into the RNA-induced silencing complex (RISC) where the miRNA and its mRNA target interact.
Biogenesis in plants
miRNA biogenesis in plants differs from metazoan biogenesis mainly in the steps of nuclear processing and export. Instead of being cleaved by two different enzymes, once inside and once outside the nucleus, both cleavages of the plant miRNA is performed by a Dicer homolog, called Dicer-like1 (DL1). DL1 is only expressed in the nucleus of plant cells, which indicates that both reactions take place inside the nucleus. Before plant miRNA:miRNA* duplexes are transported out of the nucleus its 3' overhangs are methylated by a RNA methyltransferaseprotein called Hua-Enhancer1 (HEN1). The duplex is then transported out of the nucleus to the cytoplasm by a protein called Hasty (HST), an Exportin 5 homolog, where they disassemble and the mature miRNA is incorporated into the RISC.[50]
The RNA-induced silencing complex
The mature miRNA is part of an active RNA-induced silencing complex (RISC) containing Dicer and many associated proteins.[51] RISC is also known as a microRNA ribonucleoprotein complex (miRNP);[52] RISC with incorporated miRNA is sometimes referred to as "miRISC."
Dicer processing of the pre-miRNA is thought to be coupled with unwinding of the duplex. Generally, only one strand is incorporated into the miRISC, selected on the basis of its thermodynamic instability and weaker base-pairing relative to the other strand.[53][54][55] The position of the stem-loop may also influence strand choice.[56] The other strand, called the passenger strand due to its lower levels in the steady state, is denoted with an asterisk (*) and is normally degraded. In some cases, both strands of the duplex are viable and become functional miRNA that target different mRNA populations.[57]
Members of the argonaute (Ago) protein family are central to RISC function. Argonautes are needed for miRNA-induced silencing and contain two conserved RNA binding domains: a PAZ domain that can bind the single stranded 3’ end of the mature miRNA and a PIWI domain that structurally resembles ribonuclease-H and functions to interact with the 5’ end of the guide strand. They bind the mature miRNA and orient it for interaction with a target mRNA. Some argonautes, for example human Ago2, cleave target transcripts directly; argonautes may also recruit additional proteins to achieve translational repression.[58] The human genome encodes eight argonaute proteins divided by sequence similarities into two families: AGO (with four members present in all mammalian cells and called E1F2C/hAgo in humans), and PIWI (found in the germ line and hematopoietic stem cells).[58][59]
Additional RISC components include TRBP [human immunodeficiency virus (HIV) transactivating response RNA (TAR) binding protein],[60] PACT (protein activator of the interferon induced protein kinase (PACT), the SMN complex, fragile X mental retardation protein (FMRP), and Tudor staphylococcal nuclease-domain-containing protein (Tudor-SN).[61][62]
Mode of Silencing
Gene silencing may occur either via mRNA degradation or preventing mRNA from being translated. It has been demonstrated that if there is complete complementation between the miRNA and target mRNA sequence, Ago2 can cleave the mRNA and lead to direct mRNA degradation. Yet, if there isn't complete complementation the silencing is achieved by preventing translation.[63]
miRNA turnover
Turnover of mature miRNA is needed for rapid changes in miRNA expression profiles. During miRNA maturation in the cytoplasm, uptake by the Argonaute protein is thought to stabilize the guide strand, while the opposite (* or "passenger") strand is preferentially destroyed. In what has been called a "Use it or lose it" strategy, Argonaute may preferentially retain miRNAs with many targets over miRNAs with few or no targets, leading to degradation of the non-targeting molecules.[64]
Decay of mature miRNAs in Caenorhabditis elegans is mediated by the 5´-to-3´ exoribonuclease XRN2, also known as Rat1p.[65] In plants, SDN (small RNA degrading nuclease) family members degrade miRNAs in the opposite (3'-to-5') direction. Similar enzymes are encoded in animal genomes, but their roles have not yet been described.[64]
Several miRNA modifications affect miRNA stability. As indicated by work in the model organism Arabidopsis thaliana (thale cress), mature plant miRNAs appear to be stabilized by the addition of methyl moieties at the 3' end. The 2'-O-conjugated methyl groups block the addition of uracil (U) residues by uridyltransferase enzymes, a modification that may be associated with miRNA degradation. However, uridylation may also protect some miRNAs; the consequences of this modification are incompletely understood. Uridylation of some animal miRNAs has also been reported. Both plant and animal miRNAs may be altered by addition of adenine (A) residues to the 3' end of the miRNA. An extra A added to the end of mammalian miR-122, a liver-enriched miRNA important in Hepatitis C, stabilizes the molecule, and plant miRNAs ending with an adenine residue have slower decay rates.[64]
Cellular functions
The function of miRNAs appears to be in gene regulation. For that purpose, a miRNA is complementary to a part of one or more messenger RNAs (mRNAs). Animal miRNAs are usually complementary to a site in the 3' UTR whereas plant miRNAs are usually complementary to coding regions of mRNAs.[66] Perfect or near perfect base pairing with the target RNA promotes cleavage of the RNA.[67] This is the primary mode of plant miRNAs.[68] In animals miRNAs more often have only partly the right sequence of nucleotides to bond with the target mRNA. The match-ups are imperfect. Animal miRNAs inhibit protein translation of the target mRNA[69] (this exists in plants as well but is less common).[68] MicroRNAs that are partially complementary to a target can also speed up deadenylation, causing mRNAs to be degraded sooner.[70] For partially complementary microRNAs to recognise their targets, nucleotides 2–7 of the miRNA (its 'seed region'[5][8]) still have to be perfectly complementary.[71] miRNAs occasionally also cause histone modification and DNA methylation of promoter sites, which affects the expression of target genes.[72][73]
Unlike plant microRNAs, the animal microRNAs target a diverse set of genes.[8] However, genes involved in functions common to all cells, such as gene expression, have relatively fewer microRNA target sites and seem to be under selection to avoid targeting by microRNAs.[74]
dsRNA can also activate gene expression, a mechanism that has been termed "small RNA-induced gene activation" or RNAa. dsRNAs targeting gene promoters can induce potent transcriptional activation of associated genes. This was demonstrated in human cells using synthetic dsRNAs termed small activating RNAs (saRNAs),[75] but has also been demonstrated for endogenous microRNA.[76]
Interactions between microRNAs and complementary sequences on genes and even pseudogenes that share sequence homology are thought to be a back channel of communication regulating expression levels between paralogous genes. Given the name "competing endogenous RNAs" (ceRNAs), these microRNAs bind to "microRNA response elements" on genes and pseudogenes and may provide another explanation for the persistence of non-coding ("junk") DNA.[77]
Evolution
MicroRNAs are significant phylogenetic markers because of their astonishingly low rate of evolution.[78] Their origin may have permitted the development of morphological innovation, and by making gene expression more specific and 'fine-tunable', permitted the genesis of complex organs[79] and perhaps, ultimately, complex life.[80] Indeed, rapid bursts of morphological innovation are generally associated with a high rate of microRNA accumulation.[78][79]
MicroRNAs originate predominantly by the random formation of hairpins in "non-coding" sections of DNA (i.e. introns or intergene regions), but also by the duplication and modification of existing microRNAs.[81] The rate of evolution (i.e. nucleotide substitution) in recently-originated microRNAs is comparable to that elsewhere in the non-coding DNA, implying evolution by neutral drift; however, older microRNAs have a much lower rate of change (often less than one substitution per hundred million years),[80] suggesting that once a microRNA gains a function it undergoes extreme purifying selection.[81] At this point, a microRNA is rarely lost from an animal's genome,[80] although microRNAs which are more recently derived (and thus presumably non-functional) are frequently lost.[81] This makes them a valuable phylogenetic marker, and they are being looked upon as a possible solution to such outstanding phylogenetic problems as the relationships of arthropods.[82]
MicroRNAs feature in the genomes of most eukaryotic organisms, from the brown algae[83] to the metazoa. Across all species, in excess of 5000 had been identified by March 2010.[84] Whilst short RNA sequences (50 – hundreds of base pairs) of a broadly comparable function occur in bacteria, bacteria lack true microRNAs.[85]
Experimental detection and manipulation of miRNA
MicroRNA expression can be quantified in a two-step polymerase chain reaction process of modified RT-PCR followed by quantitative real-time PCR. Variations of this method achieve absolute or relative quantification.[86] miRNAs can also be hybridized to microarrays, slides or chips with probes to hundreds or thousands of miRNA targets, so that relative levels of miRNAs can be determined in different samples.[87] MicroRNAs can be both discovered and profiled by high-throughput sequencing methods.[88] The activity of an miRNA can be experimentally inhibited using a locked nucleic acid (LNA) oligo, a Morpholino oligo[89][90] or a 2'-O-methyl RNA oligo.[91] Additionally, a specific miRNA can be silenced by a complementary antagomir. MicroRNA maturation can be inhibited at several points by steric-blocking oligos.[92] The miRNA target site of an mRNA transcript can also be blocked by a steric-blocking oligo.[93][94] For the “in situ” detection of miRNA, the use of LNA is currently the only efficient method.[95] The locked conformation of LNA results in enhanced hybridization properties and increases sensitivity and selectivity, making it ideal for detection of short miRNA.[96]
miRNA and disease
Just as miRNA is involved in the normal functioning of eukaryotic cells, so has dysregulation of miRNA been associated with disease. A manually curated, publicly available database miR2Disease documents known relationships between miRNA dysregulation and human disease.[97]
miRNA and inherited diseases
A mutation in the seed region of miR-96 causes hereditary progressive hearing loss.[98] A mutation in the seed region of miR-184 causes hereditary keratoconus with anterior polar cataract.[99] Deletion of the miR-17~92 cluster causes skeletal and growth defects.[100]
miRNA and cancer
Several miRNAs have been found to have links with some types of cancer.[101][102] MicroRNA-21 is one of the first microRNAs that was identified as an oncomiR.
A study of mice altered to produce excess c-Myc — a protein with mutated forms implicated in several cancers — shows that miRNA has an effect on the development of cancer. Mice that were engineered to produce a surplus of types of miRNA found in lymphoma cells developed the disease within 50 days and died two weeks later. In contrast, mice without the surplus miRNA lived over 100 days.[101] Leukemia can be caused by the insertion of a viral genome next to the 17-92 array of microRNAs leading to increased expression of this microRNA.[103]
Another study found that two types of miRNA inhibit the E2F1 protein, which regulates cell proliferation. miRNA appears to bind to messenger RNA before it can be translated to proteins that switch genes on and off.[104]
By measuring activity among 217 genes encoding miRNA, patterns of gene activity that can distinguish types of cancers can be discerned. miRNA signatures may enable classification of cancer. This will allow doctors to determine the original tissue type which spawned a cancer and to be able to target a treatment course based on the original tissue type. miRNA profiling has already been able to determine whether patients with chronic lymphocytic leukemia had slow growing or aggressive forms of the cancer.[105]
Transgenic mice that over-express or lack specific miRNAs have provided insight into the role of small RNAs in various malignancies.[106]
A novel miRNA-profiling based screening assay for the detection of early-stage colorectal cancer has been developed and is currently in clinical trials. Early results showed that blood plasma samples collected from patients with early, resectable (Stage II) colorectal cancer could be distinguished from those of sex-and age-matched healthy volunteers. Sufficient selectivity and specificity could be achieved using small (less than 1 mL) samples of blood. The test has potential to be a cost-effective, non-invasive way to identify at-risk patients who should undergo colonoscopy.[107][108]
Another role for miRNA in cancers is to use their expression level as a prognostic, for example one study on NSCLC samples found that low miR-324a levels could serve as a prognostic indicator of poor survival,[109] another found that either high miR-185 or low miR-133b levels correlated with metastasis and poor survival in colorectal cancer.[110]
miRNA and heart disease
The global role of miRNA function in the heart has been addressed by conditionally inhibiting miRNA maturation in the murine heart, and has revealed that miRNAs play an essential role during its development.[111][112] miRNA expression profiling studies demonstrate that expression levels of specific miRNAs change in diseased human hearts, pointing to their involvement in cardiomyopathies.[113][114][115] Furthermore, studies on specific miRNAs in animal models have identified distinct roles for miRNAs both during heart development and under pathological conditions, including the regulation of key factors important for cardiogenesis, the hypertrophic growth response, and cardiac conductance.[112][116][117][118][119][120]
miRNA and the nervous system
miRNAs appear to regulate the nervous system.[121] Neural miRNAs are involved at various stages of synaptic development, including dendritogenesis (involving miR-132, miR-134 and miR-124), synapse formation and synapse maturation (where miR-134 and miR-138 are thought to be involved).[122] Some studies find altered miRNA expression in schizophrenia.[123][124]
miRNA and non-coding RNAs
When the human genome project mapped its first chromosome in 1999, it was predicted the genome would contain over 100,000 protein coding genes. However, only around 20,000 were eventually identified (International Human Genome Sequencing Consortium, 2004).[125] Since then, the advent of bioinformatics approaches combined with genome tiling studies examining the transcriptome,[126] systematic sequencing of full length cDNA libraries,[127] and experimental validation[128] (including the creation of miRNA derived antisense oligonucleotides called antagomirs) have revealed that many transcripts are non protein-coding RNA, including several snoRNAs and miRNAs.[129]
See also
- Gene expression
- RNAi
- siRNA
- List of miRNA target prediction tools
- List of miRNA gene prediction tools
References
- ^ Bartel, D. P. (2009). "MicroRNAs: target recognition and regulatory functions". Cell 136 (2): 215–233. doi:10.1016/j.cell.2009.01.002. PMID 19167326.
- ^ Bartel DP (January 2004). "MicroRNAs: genomics, biogenesis, mechanism, and function". Cell 116 (2): 281–97. doi:10.1016/S0092-8674(04)00045-5. PMID 14744438.
- ^ Homo sapiens miRNAs in the miRBase at Manchester University
- ^ Bentwich I, Avniel A, Karov Y, Aharonov R, Gilad S, Barad O, Barzilai A, Einat P, Einav U, Meiri E, Sharon E, Spector Y, Bentwich Z (July 2005). "Identification of hundreds of conserved and nonconserved human microRNAs". Nat. Genet. 37 (7): 766–70. doi:10.1038/ng1590. PMID 15965474.
- ^ a b c Lewis BP, Burge CB, Bartel DP (2005). "Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets". Cell 120 (1): 15–20. doi:10.1016/j.cell.2004.12.035. PMID 15652477.
- ^ Friedman RC, Farh KK, Burge CB, Bartel DP (January 2009). "Most mammalian mRNAs are conserved targets of microRNAs". Genome Res. 19 (1): 92–105. doi:10.1101/gr.082701.108. PMC 2612969. PMID 18955434. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2612969.
- ^ Lim LP, Lau NC, Weinstein EG, Abdelhakim A, Yekta S, Rhoades MW, Burge CB, Bartel DP (April 2003). "The microRNAs of Caenorhabditis elegans". Genes Dev. 17 (8): 991–1008. doi:10.1101/gad.1074403. PMC 196042. PMID 12672692. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=196042.
- ^ a b c Lewis BP, Shih IH, Jones-Rhoades M, Bartel DP, Burge CB (2003). "Prediction of Mammalian MicroRNA Targets". Cell 115 (7): 787–798. doi:10.1016/S0092-8674(03)01018-3. PMID 14697198.
- ^ He L, Hannon GJ (July 2004). "MicroRNAs: small RNAs with a big role in gene regulation". Nat. Rev. Genet. 5 (7): 522–31. doi:10.1038/nrg1379. PMID 15211354.
- ^ Tanzer A, Stadler PF (May 2004). "Molecular evolution of a microRNA cluster". J. Mol. Biol. 339 (2): 327–35. doi:10.1016/j.jmb.2004.03.065. PMID 15136036.
- ^ Molnár A, Schwach F, Studholme DJ, Thuenemann EC, Baulcombe DC (June 2007). "miRNAs control gene expression in the single-cell alga Chlamydomonas reinhardtii". Nature 447 (7148): 1126–9. doi:10.1038/nature05903. PMID 17538623.
- ^ Kren BT, Wong PY, Sarver A, Zhang X, Zeng Y, Steer CJ (2009). "MicroRNAs identified in highly purified liver-derived mitochondria may play a role in apoptosis". RNA Biol 6 (1): 65–72. doi:10.4161/rna.6.1.7534. PMID 19106625.
- ^ Lee CT, Risom T, Strauss WM (April 2007). "Evolutionary conservation of microRNA regulatory circuits: an examination of microRNA gene complexity and conserved microRNA-target interactions through metazoan phylogeny". DNA Cell Biol. 26 (4): 209–18. doi:10.1089/dna.2006.0545. PMID 17465887.
- ^ Lim LP, Lau NC, Garrett-Engele P, Grimson A, Schelter JM, Castle J, Bartel DP, Linsley PS, Johnson JM (February 2005). "Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs". Nature 433 (7027): 769–73. doi:10.1038/nature03315. PMID 15685193.
- ^ Brennecke J, Hipfner DR, Stark A, Russell RB, Cohen SM (April 2003). "bantam encodes a developmentally regulated microRNA that controls cell proliferation and regulates the proapoptotic gene hid in Drosophila". Cell 113 (1): 25–36. doi:10.1016/S0092-8674(03)00231-9. PMID 12679032.
- ^ Cuellar TL, McManus MT (December 2005). "MicroRNAs and endocrine biology". J. Endocrinol. 187 (3): 327–32. doi:10.1677/joe.1.06426. PMID 16423811.
- ^ Poy MN, Eliasson L, Krutzfeldt J, Kuwajima S, Ma X, Macdonald PE, Pfeffer S, Tuschl T, Rajewsky N, Rorsman P, Stoffel M (November 2004). "A pancreatic islet-specific microRNA regulates insulin secretion". Nature 432 (7014): 226–30. doi:10.1038/nature03076. PMID 15538371.
- ^ Chen CZ, Li L, Lodish HF, Bartel DP (January 2004). "MicroRNAs modulate hematopoietic lineage differentiation". Science 303 (5654): 83–6. doi:10.1126/science.1091903. PMID 14657504.
- ^ Wilfred BR, Wang WX, Nelson PT (July 2007). "Energizing miRNA research: a review of the role of miRNAs in lipid metabolism, with a prediction that miR-103/107 regulates human metabolic pathways". Mol. Genet. Metab. 91 (3): 209–17. doi:10.1016/j.ymgme.2007.03.011. PMC 1978064. PMID 17521938. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1978064.
- ^ Harfe BD, McManus MT, Mansfield JH, Hornstein E, Tabin CJ (August 2005). "The RNaseIII enzyme Dicer is required for morphogenesis but not patterning of the vertebrate limb". Proc. Natl. Acad. Sci. U.S.A. 102 (31): 10898–903. doi:10.1073/pnas.0504834102. PMC 1182454. PMID 16040801. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1182454.
- ^ Lagos-Quintana M, Rauhut R, Yalcin A, Meyer J, Lendeckel W, Tuschl T (April 2002). "Identification of tissue-specific microRNAs from mouse". Curr. Biol. 12 (9): 735–9. doi:10.1016/S0960-9822(02)00809-6. PMID 12007417.
- ^ Trang, P.; Weidhaas, J. B.; Slack, F. J. (2008). "MicroRNAs as potential cancer therapeutics". Oncogene 27 Suppl 2: S52–S57. doi:10.1038/onc.2009.353. PMID 19956180.
- ^ Li, C.; Feng, Y.; Coukos, G.; Zhang, L. (2009). "Therapeutic MicroRNA Strategies in Human Cancer". The AAPS journal 11 (4): 747–757. doi:10.1208/s12248-009-9145-9. PMC 2782079. PMID 19876744. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2782079.
- ^ Fasanaro, P.; Greco, S.; Ivan, M.; Capogrossi, M.; Martelli, F. (2010). "MicroRNA: emerging therapeutic targets in acute ischemic diseases". Pharmacology & therapeutics 125 (1): 92–104. doi:10.1016/j.pharmthera.2009.10.003. PMID 19896977.
- ^ Lee RC, Feinbaum RL, Ambros V (December 1993). "The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14". Cell 75 (5): 843–54. doi:10.1016/0092-8674(93)90529-Y. PMID 8252621.
- ^ Reinhart BJ, Slack FJ, Basson M, Pasquinelli AE, Bettinger JC, Rougvie AE, Horvitz HR, Ruvkun G (February 2000). "The 21-nucleotide let-7 RNA regulates developmental timing in Caenorhabditis elegans". Nature 403 (6772): 901–6. Bibcode 2000Natur.403..901R. doi:10.1038/35002607. PMID 10706289.
- ^ Pasquinelli AE, Reinhart BJ, Slack F, Martindale MQ, Kuroda MI, Maller B, Hayward DC, Ball EE, Degnan B, Müller P, Spring J, Srinivasan A, Fishman M, Finnerty J, Corbo J, Levine M, Leahy P, Davidson E, Ruvkun G (November 2000). "Conservation of the sequence and temporal expression of let-7 heterochronic regulatory RNA". Nature 408 (6808): 86–9. doi:10.1038/35040556. PMID 11081512.
- ^ Ambros V, Bartel B, Bartel DP, Burge CB, Carrington JC, Chen X, Dreyfuss G, Eddy SR, Griffiths-Jones S, Marshall M, Matzke M, Ruvkun G, Tuschl T (March 2003). "A uniform system for microRNA annotation". RNA 9 (3): 277–9. doi:10.1261/rna.2183803. PMC 1370393. PMID 12592000. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1370393.
- ^ Griffiths-Jones S, Grocock RJ, van Dongen S, Bateman A, Enright AJ (January 2006). "miRBase: microRNA sequences, targets and gene nomenclature". Nucleic Acids Res. 34 (Database issue): D140–4. doi:10.1093/nar/gkj112. PMC 1347474. PMID 16381832. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1347474.
- ^ a b Lau NC, Lim LP, Weinstein EG, Bartel DP (October 2001). "An abundant class of tiny RNAs with probable regulatory roles in Caenorhabditis elegans". Science 294 (5543): 858–62. doi:10.1126/science.1065062. PMID 11679671.
- ^ a b c d e f Lee Y, Kim M, Han J, Yeom KH, Lee S, Baek SH, Kim VN (October 2004). "MicroRNA genes are transcribed by RNA polymerase II". EMBO J. 23 (20): 4051–60. doi:10.1038/sj.emboj.7600385. PMC 524334. PMID 15372072. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=524334.
- ^ Lagos-Quintana M, Rauhut R, Lendeckel W, Tuschl T (October 2001). "Identification of novel genes coding for small expressed RNAs". Science 294 (5543): 853–8. doi:10.1126/science.1064921. PMID 11679670.
- ^ Lee RC, Ambros V (October 2001). "An extensive class of small RNAs in Caenorhabditis elegans". Science 294 (5543): 862–4. doi:10.1126/science.1065329. PMID 11679672.
- ^ a b Rodriguez A, Griffiths-Jones S, Ashurst JL, Bradley A (October 2004). "Identification of mammalian microRNA host genes and transcription units". Genome Res. 14 (10A): 1902–10. doi:10.1101/gr.2722704. PMC 524413. PMID 15364901. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=524413.
- ^ a b c d Cai X, Hagedorn CH, Cullen BR (December 2004). "Human microRNAs are processed from capped, polyadenylated transcripts that can also function as mRNAs". RNA 10 (12): 1957–66. doi:10.1261/rna.7135204. PMC 1370684. PMID 15525708. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1370684.
- ^ Weber MJ (January 2005). "New human and mouse microRNA genes found by homology search". FEBS J. 272 (1): 59–73. doi:10.1111/j.1432-1033.2004.04389.x. PMID 15634332.
- ^ Kim YK, Kim VN (February 2007). "Processing of intronic microRNAs". EMBO J. 26 (3): 775–83. doi:10.1038/sj.emboj.7601512. PMC 1794378. PMID 17255951. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1794378.
- ^ Baskerville S, Bartel DP (March 2005). "Microarray profiling of microRNAs reveals frequent coexpression with neighboring miRNAs and host genes". RNA 11 (3): 241–7. doi:10.1261/rna.7240905. PMC 1370713. PMID 15701730. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1370713.
- ^ Altuvia Y, Landgraf P, Lithwick G, Elefant N, Pfeffer S, Aravin A, Brownstein MJ, Tuschl T, Margalit H (2005). "Clustering and conservation patterns of human microRNAs". Nucleic Acids Res. 33 (8): 2697–706. doi:10.1093/nar/gki567. PMC 1110742. PMID 15891114. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1110742.
- ^ a b Zhou X, Ruan J, Wang G, Zhang W (March 2007). "Characterization and identification of microRNA core promoters in four model species". PLoS Comput. Biol. 3 (3): e37. doi:10.1371/journal.pcbi.0030037. PMC 1817659. PMID 17352530. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1817659.
- ^ Faller, M.; Guo, F. (2008). "MicroRNA biogenesis: there's more than one way to skin a cat.". Biochimica et Biophysica Acta 1779 (11): 663–667. doi:10.1016/j.bbagrm.2008.08.005. PMC 2633599. PMID 18778799. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2633599.
- ^ Gregory, R.; Chendrimada, T.; Shiekhattar, R. (2006). "MicroRNA biogenesis: isolation and characterization of the microprocessor complex". Methods in molecular biology (Clifton, N.J.) 342: 33–48. doi:10.1385/1-59745-123-1:33. ISBN 1-59745-123-1. PMID 16957365.
- ^ Berezikov, E.; Chung, W.; Willis, J.; Cuppen, E.; Lai, E. (2007). "Mammalian mirtron genes". Molecular cell 28 (2): 328–336. doi:10.1016/j.molcel.2007.09.028. PMC 2763384. PMID 17964270. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2763384.
- ^ a b Kawahara, Y.; Megraw, M.; Kreider, E.; Iizasa, H.; Valente, L.; Hatzigeorgiou, A.; Nishikura, K. (2008). "Frequency and fate of microRNA editing in human brain". Nucleic Acids Research 36 (16): 5270–5280. doi:10.1093/nar/gkn479. PMC 2532740. PMID 18684997. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2532740.
- ^ Winter, J.; Jung, S.; Keller, S.; Gregory, R.; Diederichs, S. (2009). "Many roads to maturity: microRNA biogenesis pathways and their regulation". Nature cell biology 11 (3): 228–234. doi:10.1038/ncb0309-228. PMID 19255566.
- ^ Ohman, M. (2007). "A-to-I editing challenger or ally to the microRNA process". Biochimie 89 (10): 1171–1176. doi:10.1016/j.biochi.2007.06.002. PMID 17628290.
- ^ Murchison, E.; Hannon, G. (2004). "MiRNAs on the move: miRNA biogenesis and the RNAi machinery". Current opinion in cell biology 16 (3): 223–229. doi:10.1016/j.ceb.2004.04.003. PMID 15145345.
- ^ a b c Lund, E.; Dahlberg, J. (2006). "Substrate selectivity of exportin 5 and Dicer in the biogenesis of microRNAs". Cold Spring Harbor symposia on quantitative biology 71: 59–66. doi:10.1101/sqb.2006.71.050. PMID 17381281.
- ^ Ji, X. (2008). "The mechanism of RNase III action: how dicer dices". Current topics in microbiology and immunology 320: 99–116. doi:10.1007/978-3-540-75157-1_5. PMID 18268841.
- ^ Lelandais-Brière C, Sorin C, Declerck M, Benslimane A, Crespi M, Hartmann C (March 2010). "Small RNA diversity in plants and its impact in development". Current Genomics 11 (1): 14–23. doi:10.2174/138920210790217918. PMC 2851111. PMID 20808519. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2851111.
- ^ Rana, T. M. (2007). "Illuminating the silence: understanding the structure and function of small RNAs". Nature Reviews Molecular Cell Biology 8 (1): 23–36. doi:10.1038/nrm2085. PMID 17183358.
- ^ Schwarz, D.; Zamore, P. (2002). "Why do miRNAs live in the miRNP?". Genes & development 16 (9): 1025–1031. doi:10.1101/gad.992502. PMID 12000786.
- ^ Krol J, Sobczak K, Wilczynska U, Drath M, Jasinska A, Kaczynska D, Krzyzosiak WJ (2004). "Structural features of microRNA (miRNA) precursors and their relevance to miRNA biogenesis and small interfering RNA/short hairpin RNA design.". J Biol Chem 279 (40): 42230–9. doi:10.1074/jbc.M404931200. PMID 15292246.
- ^ Khvorova A, Reynolds A, Jayasena SD (2003). "Functional siRNAs and miRNAs exhibit strand bias.". Cell 115 (2): 209–16. doi:10.1016/S0092-8674(03)00801-8. PMID 14567918.
- ^ Schwarz DS, Hutvágner G, Du T, Xu Z, Aronin N, Zamore PD (2003). "Asymmetry in the assembly of the RNAi enzyme complex.". Cell 115 (2): 199–208. doi:10.1016/S0092-8674(03)00759-1. PMID 14567917.
- ^ Lin SL, Chang D, Ying SY (2005). "Asymmetry of intronic pre-miRNA structures in functional RISC assembly.". Gene 356: 32–8. doi:10.1016/j.gene.2005.04.036. PMC 1788082. PMID 16005165. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1788082.
- ^ Okamura K, Chung WJ, Lai EC (2008). "The long and short of inverted repeat genes in animals: microRNAs, mirtrons and hairpin RNAs.". Cell Cycle 7 (18): 2840–5. doi:10.4161/cc.7.18.6734. PMC 2697033. PMID 18769156. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2697033.
- ^ a b Pratt, A.; MacRae, I. (2009). "The RNA-induced silencing complex: a versatile gene-silencing machine". The Journal of biological chemistry 284 (27): 17897–17901. doi:10.1074/jbc.R900012200. PMC 2709356. PMID 19342379. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2709356.
- ^ Schwarz, D.; Zamore, P. (2002). "Why do miRNAs live in the miRNP?". Genes & development 16 (9): 1025–1031. doi:10.1101/gad.992502. PMID 12000786.
- ^ MacRae, I.; Ma, E.; Zhou, M.; Robinson, C.; Doudna, J. (2008). "In vitro reconstitution of the human RISC-loading complex". Proceedings of the National Academy of Sciences of the United States of America 105 (2): 512–517. Bibcode 2008PNAS..105..512M. doi:10.1073/pnas.0710869105. PMC 2206567. PMID 18178619. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2206567.
- ^ Murchison, E.; Hannon, G. (2004). "MiRNAs on the move: miRNA biogenesis and the RNAi machinery". Current opinion in cell biology 16 (3): 223–229. doi:10.1016/j.ceb.2004.04.003. PMID 15145345.
- ^ Mourelatos Z, Dostie J, Paushkin S, et al. (March 2002). "miRNPs: a novel class of ribonucleoproteins containing numerous microRNAs". Genes Dev. 16 (6): 720–8. doi:10.1101/gad.974702. PMC 155365. PMID 11914277. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=155365.
- ^ Lim, Lee; Nelson C. Lau, Philip Garrett-Engele, Andrew Grimson, Janell M. Schelter, John Castle, David P. Bartel, Peter S. Linsley, Jason M. Johnson (17). "Microarray analysis shows that some microRNAs downregulate large number of target mRNAs". Nature 433 (7027): 769–773. doi:10.1038/nature03315. PMID 15685193.
- ^ a b c Kai, Z.; Pasquinelli, A. (2010). "MicroRNA assassins: factors that regulate the disappearance of miRNAs". Nature structural & molecular biology 17 (1): 5–10. doi:10.1038/nsmb.1762. PMID 20051982.
- ^ Chatterjee S, Großhans H (September 2009). "Active turnover modulates mature microRNA activity in Caenorhabditis elegans". Nature 461 (7263): 546–459. doi:10.1038/nature08349. PMID 19734881.
- ^ Wang XJ, Reyes JL, Chua NH, Gaasterland T (2004). "Prediction and identification of Arabidopsis thaliana microRNAs and their mRNA targets". Genome Biol. 5 (9): R65. doi:10.1186/gb-2004-5-9-r65. PMC 522872. PMID 15345049. http://genomebiology.com/2004/5/9/R65.
- ^ Kawasaki H, Taira K (2004). "MicroRNA-196 inhibits HOXB8 expression in myeloid differentiation of HL60 cells". Nucleic Acids Symp Ser 48 (48): 211–2. doi:10.1093/nass/48.1.211. PMID 17150553.
- ^ a b Moxon S, Jing R, Szittya G, et al. (October 2008). "Deep sequencing of tomato short RNAs identifies microRNAs targeting genes involved in fruit ripening". Genome Res. 18 (10): 1602–9. doi:10.1101/gr.080127.108. PMC 2556272. PMID 18653800. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2556272.
- ^ Williams AE (February 2008). "Functional aspects of animal microRNAs". Cell. Mol. Life Sci. 65 (4): 545–62. doi:10.1007/s00018-007-7355-9. PMID 17965831.
- ^ Eulalio A, Huntzinger E, Nishihara T, Rehwinkel J, Fauser M, Izaurralde E (January 2009). "Deadenylation is a widespread effect of miRNA regulation". RNA 15 (1): 21–32. doi:10.1261/rna.1399509. PMC 2612776. PMID 19029310. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2612776.
- ^ Mazière P, Enright AJ (June 2007). "Prediction of microRNA targets". Drug Discov. Today 12 (11-12): 452–8. doi:10.1016/j.drudis.2007.04.002. PMID 17532529.
- ^ Tan Y, Zhang B, Wu T, et al. (February 2009). "Transcriptional inhibition of Hoxd4 expression by noncoding RNAs in human breast cancer cells". BMC Mol. Biol. 10: 12. doi:10.1186/1471-2199-10-12. PMC 2680403. PMID 19232136. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2680403.
- ^ Hawkins PG, Morris KV (March 2008). "RNA and transcriptional modulation of gene expression". Cell Cycle 7 (5): 602–7. doi:10.4161/cc.7.5.5522. PMC 2877389. PMID 18256543. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2877389.
- ^ Stark A, Brennecke J, Bushati N, Russell RB, Cohen SM (2005). "Animal MicroRNAs confer robustness to gene expression and have a significant impact on 3'UTR evolution". Cell 123 (6): 1133–46. doi:10.1016/j.cell.2005.11.023. PMID 16337999.
- ^ Li LC (2008). "Small RNA-Mediated Gene Activation". RNA and the Regulation of Gene Expression: A Hidden Layer of Complexity. Caister Academic Press. ISBN 978-1-904455-25-7. http://www.horizonpress.com/rnareg.
- ^ Place RF, Li LC, Pookot D, Noonan EJ, Dahiya R (2008). "MicroRNA-373 induces expression of genes with complementary promoter sequences". Proc. Natl. Acad. Sci. U.S.A. 105 (5): 1608–13. doi:10.1073/pnas.0707594105. PMC 2234192. PMID 18227514. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2234192.
- ^ Salmena, Leonardo; Poliseno, Laura; Tay, Yvonne; Kats, Lev; Pandolfi, Pier Paolo (2011). "A ceRNA Hypothesis: The Rosetta Stone of a Hidden RNA Language?". Cell (Elsevier Inc) 146 (3): 353–358. doi:10.1016/j.cell.2011.07.014. http://www.cell.com/abstract/S0092-8674(11)00812-9. Retrieved 15 August 2011.
- ^ a b Wheeler, B.; Heimberg, A.; Moy, V.; Sperling, E.; Holstein, T.; Heber, S.; Peterson, K. (2009). "The deep evolution of metazoan microRNAs". Evolution & development 11 (1): 50–68. doi:10.1111/j.1525-142X.2008.00302.x. PMID 19196333.
- ^ a b Heimberg, A.; Sempere, L.; Moy, V.; Donoghue, P.; Peterson, K. (2008). "MicroRNAs and the advent of vertebrate morphological complexity". Proceedings of the National Academy of Sciences of the United States of America 105 (8): 2946–2950. Bibcode 2008PNAS..105.2946H. doi:10.1073/pnas.0712259105. PMC 2268565. PMID 18287013. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2268565.
- ^ a b c Peterson, K.; Dietrich, M.; McPeek, M. (2009). "MicroRNAs and metazoan macroevolution: insights into canalization, complexity, and the Cambrian explosion". BioEssays : news and reviews in molecular, cellular and developmental biology 31 (7): 736–747. doi:10.1002/bies.200900033. PMID 19472371.
- ^ a b c Nozawa, M.; Miura, S.; Nei, M. (2010). "Origins and Evolution of MicroRNA Genes in Drosophila Species". Genome Biology and Evolution 2010: 180–189. doi:10.1093/gbe/evq009. PMC 2942034. PMID 20624724. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2942034.
- ^ Caravas, J.; Friedrich, M. (2010). "Of mites and millipedes: Recent progress in resolving the base of the arthropod tree". BioEssays 32 (6): 488. doi:10.1002/bies.201000005. PMID 20486135.
- ^ Cock, J. M.; Sterck, L.; Rouzé, P.; Scornet, D.; Allen, A. E.; Amoutzias, G.; Anthouard, V.; Artiguenave, F. O. et al. (2010). "The Ectocarpus genome and the independent evolution of multicellularity in brown algae". Nature 465 (7298): 617–621. Bibcode 2010Natur.465..617C. doi:10.1038/nature09016. PMID 20520714.
- ^ Dimond, Patricia F. (15 March 2010). "miRNAs' Therapeutic Potential". Genetic Engineering & Biotechnology News 30 (6): p. 1. Archived from the original on 10 July 2010. http://www.webcitation.org/5r7L7BfnG. Retrieved 10 July 2010
- ^ Tjaden, B.; Goodwin, S. S.; Opdyke, J. A.; Guillier, M.; Fu, D. X.; Gottesman, S.; Storz, G. (2006). "Target prediction for small, noncoding RNAs in bacteria". Nucleic Acids Research 34 (9): 2791. doi:10.1093/nar/gkl356. PMC 1464411. PMID 16717284. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1464411.
- ^ Caifu, Chen; Dana A. Ridzon, Adam J. Broomer, Zhaohui Zhou, Danny H. Lee, Julie T. Nguyen, Maura Barbisin, Nan Lan Xu, Vikram R. Mahuvakar, Mark R. Andersen, Kai Qin Lao, Kenneth J. Livak and Karl J. Guegler (2005-10-25). "Real-time quantification of microRNAs by stem–loop RT–PCR". Nucleic Acids Research 33 (20): e179. doi:10.1093/nar/gni178. PMC 1292995. PMID 16314309. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1292995.
- ^ Shingara J, Keiger K, Shelton J, Laosinchai-Wolf W, Powers P, Conrad R, Brown D, Labourier E (September 2005). "An optimized isolation and labeling platform for accurate microRNA expression profiling". RNA 11 (9): 1461–70. doi:10.1261/rna.2610405. PMC 1370829. PMID 16043497. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1370829.
- ^ Buermans HP, Ariyurek Y, van Ommen G, den Dunnen JT, 't Hoen PA. (December 2010). "New methods for next generation sequencing based microRNA expression profiling". BMC Genomics 11: 716. doi:10.1186/1471-2164-11-716. PMC 3022920. PMID 21171994. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=3022920.
- ^ Kloosterman WP, Wienholds E, Ketting RF, Plasterk RH (2004). "Substrate requirements for let-7 function in the developing zebrafish embryo". Nucleic Acids Res. 32 (21): 6284–91. doi:10.1093/nar/gkh968. PMC 535676. PMID 15585662. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=535676.
- ^ Flynt AS, Li N, Thatcher EJ, Solnica-Krezel L, Patton JG (February 2007). "Zebrafish miR-214 modulates Hedgehog signaling to specify muscle cell fate". Nat. Genet. 39 (2): 259–63. doi:10.1038/ng1953. PMID 17220889.
- ^ Meister G, Landthaler M, Dorsett Y, Tuschl T (March 2004). "Sequence-specific inhibition of microRNA- and siRNA-induced RNA silencing". RNA 10 (3): 544–50. doi:10.1261/rna.5235104. PMC 1370948. PMID 14970398. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1370948.
- ^ Kloosterman WP, Lagendijk AK, Ketting RF, Moulton JD, Plasterk RH (August 2007). "Targeted inhibition of miRNA maturation with morpholinos reveals a role for miR-375 in pancreatic islet development". PLoS Biol. 5 (8): e203. doi:10.1371/journal.pbio.0050203. PMC 1925136. PMID 17676975. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1925136.
- ^ Choi, WY; Giraldez AJ, Schier AF (2007). "Target Protectors Reveal Dampening and Balancing of Nodal Agonist and Antagonist by miR-430.". Science. 318 (5848): 271–4. doi:10.1126/science.1147535. PMID 17761850.
- ^ Klein, E.; Lioy, T.; Ma, L.; Impey, S.; Mandel, G.; Goodman, H. (Dec 2007). "Homeostatic regulation of MeCP2 expression by a CREB-induced microRNA". Nature neuroscience 10 (12): 1513–1514. doi:10.1038/nn2010. ISSN 1097-6256. PMID 17994015.
- ^ You Y, Moreira BG, Behlke MA, Owczarzy R (2006). "Design of LNA probes that improve mismatch discrimination". Nucleic Acids Res 34 (8): e60. doi:10.1093/nar/gkl175. PMC 1456327. PMID 16670427. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1456327.
- ^ Kaur H, Arora A, Wengel J, Maiti S, Arora A, Wengel J, Maiti S (2006). "Thermodynamic, Counterion, and Hydration Effects for the Incorporation of Locked Nucleic Acid Nucleotides into DNA Duplexes". Biochemistry 45 (23): 7347–55. doi:10.1021/bi060307w. PMID 16752924.
- ^ Jiang Q, Wang Y, Hao Y, Juan L, Teng M, Zhang X, Li M, Wang G, Liu Y. (January 2009). "miR2Disease: a manually curated database for microRNA deregulation in human disease.". Nucleic Acids Research. 37 (Database issue) (Database issue): D98–104. doi:10.1093/nar/gkn714. PMC 2686559. PMID 18927107. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2686559.
- ^ Mencía, Á.; Modamio-Høybjør, S.; Redshaw, N.; Morín, M. A.; Mayo-Merino, F.; Olavarrieta, L.; Aguirre, L. A.; Del Castillo, I. et al. (2009). "Mutations in the seed region of human miR-96 are responsible for nonsyndromic progressive hearing loss". Nature Genetics 41 (5): 609–613. doi:10.1038/ng.355. PMID 19363479.
- ^ Hughes AE, Bradley DT, Campbell M, Lechner J, Dash DP, Simpson DA, Willoughby CE (2011). "Mutation Altering the miR-184 Seed Region Causes Familial Keratoconus with Cataract". The American Journal of Human Genetics. doi:10.1016/j.ajhg.2011.09.014. http://www.cell.com/AJHG/abstract/S0002-9297(11)00404-6. Retrieved 14/10/2011.
- ^ De Pontual, L. C.; Yao, E.; Callier, P.; Faivre, L.; Drouin, V. R.; Cariou, S.; Van Haeringen, A.; Geneviève, D. et al. (2011). "Germline deletion of the miR-17-92 cluster causes growth and skeletal defects in humans". Nature Genetics 43 (10): 1026–1030. doi:10.1038/ng.915. PMC 3184212. PMID 21892160. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=3184212.
- ^ a b He L, Thomson JM, Hemann MT, et al. (June 2005). "A microRNA polycistron as a potential human oncogene". Nature 435 (7043): 828–33. doi:10.1038/nature03552. PMID 15944707.
- ^ Mraz M, Pospisilova S, Malinova K, et al. (March 2009). "MicroRNAs in chronic lymphocytic leukemia pathogenesis and disease subtypes". Leuk Lymphoma 50 (3): 506–9. doi:10.1080/10428190902763517. PMID 19347736.
- ^ Cui JW, Li YJ, Sarkar A, Brown J, Tan YH, Premyslova M, Michaud C, Iscove N, Wang GJ, Ben-David Y. (June 2007). "Retroviral insertional activation of the Fli-3 locus in erythroleukemias encoding a cluster of microRNAs that convert Epo-induced differentiation to proliferation.". Blood 110 (7): 2631–40. doi:10.1182/blood-2006-10-053850. PMID 17586726.
- ^ O'Donnell KA, Wentzel EA, Zeller KI, Dang CV, Mendell JT (June 2005). "c-Myc-regulated microRNAs modulate E2F1 expression". Nature 435 (7043): 839–43. doi:10.1038/nature03677. PMID 15944709.
- ^ Lu J, Getz G, Miska EA, et al. (June 2005). "MicroRNA expression profiles classify human cancers". Nature 435 (7043): 834–8. doi:10.1038/nature03702. PMID 15944708.
- ^ Zanesi, N; et al. (2010). "MicroRNAs in mouse models of lymphoid malignancies.". J Nucl Acid Invest 1 (8): 36–40. doi:10.4081/jnai.2010.e8.
- ^ "Screening Tool Can Detect Colorectal Cancer from a Small Blood Sample" (Press release). American Association for Cancer Research. 2010-09-29. http://www.aacr.org/home/public--media/aacr-press-releases.aspx?d=2068. Retrieved 2010-11-29.
- ^ Nielsen BS, Jørgensen S, Fog JU, Søkilde R, Christensen IJ, Hansen U, Brünner N, Baker A, Møller S, Nielsen HJ (October 2010). "High levels of microRNA-21 in the stroma of colorectal cancers predict short disease-free survival in stage II colon cancer patients". Clin Exp Metastasis 28 (1): 27–38. doi:10.1007/s10585-010-9355-7. PMC 2998639. PMID 21069438. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2998639.
- ^ Võsa U, Vooder T, Kolde R, et al. (October 2011). "Identification of miR-374a as a prognostic marker for survival in patients with early-stage nonsmall cell lung cancer". Genes Chromosomes Cancer 50 (1): 812–22. doi:10.1002/gcc.20902. PMID 21748820.
- ^ Akçakaya P, Ekelund S, Kolosenko I, et al. (August 2011). "miR-185 and miR-133b deregulation is associated with overall survival and metastasis in colorectal cancer". Int J Oncol 39 (2): 311–8. doi:10.3892/ijo.2011.1043. PMID 21573504.
- ^ Chen JF, Murchison EP, Tang R, et al. (February 2008). "Targeted deletion of Dicer in the heart leads to dilated cardiomyopathy and heart failure". Proc. Natl. Acad. Sci. U.S.A. 105 (6): 2111–6. doi:10.1073/pnas.0710228105. PMC 2542870. PMID 18256189. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2542870.
- ^ a b Zhao Y, Ransom JF, Li A, et al. (April 2007). "Dysregulation of cardiogenesis, cardiac conduction, and cell cycle in mice lacking miRNA-1-2". Cell 129 (2): 303–17. doi:10.1016/j.cell.2007.03.030. PMID 17397913.
- ^ Thum T, Galuppo P, Wolf C, et al. (July 2007). "MicroRNAs in the human heart: a clue to fetal gene reprogramming in heart failure". Circulation 116 (3): 258–67. doi:10.1161/CIRCULATIONAHA.107.687947. PMID 17606841.
- ^ van Rooij E, Sutherland LB, Liu N, et al. (November 2006). "A signature pattern of stress-responsive microRNAs that can evoke cardiac hypertrophy and heart failure". Proc. Natl. Acad. Sci. U.S.A. 103 (48): 18255–60. doi:10.1073/pnas.0608791103. PMC 1838739. PMID 17108080. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1838739.
- ^ Tatsuguchi M, Seok HY, Callis TE, et al. (June 2007). "Expression of microRNAs is dynamically regulated during cardiomyocyte hypertrophy". J. Mol. Cell. Cardiol. 42 (6): 1137–41. doi:10.1016/j.yjmcc.2007.04.004. PMC 1934409. PMID 17498736. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1934409.
- ^ Zhao Y, Samal E, Srivastava D (July 2005). "Serum response factor regulates a muscle-specific microRNA that targets Hand2 during cardiogenesis". Nature 436 (7048): 214–20. doi:10.1038/nature03817. PMID 15951802.
- ^ Xiao J, Luo X, Lin H, et al. (April 2007). "MicroRNA miR-133 represses HERG K+ channel expression contributing to QT prolongation in diabetic hearts". J. Biol. Chem. 282 (17): 12363–7. doi:10.1074/jbc.C700015200. PMID 17344217. http://www.jbc.org/cgi/pmidlookup?view=long&pmid=17344217.
- ^ Yang B, Lin H, Xiao J, et al. (April 2007). "The muscle-specific microRNA miR-1 regulates cardiac arrhythmogenic potential by targeting GJA1 and KCNJ2". Nat. Med. 13 (4): 486–91. doi:10.1038/nm1569. PMID 17401374.
- ^ Carè A, Catalucci D, Felicetti F, et al. (May 2007). "MicroRNA-133 controls cardiac hypertrophy". Nat. Med. 13 (5): 613–8. doi:10.1038/nm1582. PMID 17468766.
- ^ van Rooij E, Sutherland LB, Qi X, Richardson JA, Hill J, Olson EN (April 2007). "Control of stress-dependent cardiac growth and gene expression by a microRNA". Science 316 (5824): 575–9. doi:10.1126/science.1139089. PMID 17379774.
- ^ Maes OC, Chertkow HM, Wang E, Schipper HM (May 2009). "MicroRNA: Implications for Alzheimer Disease and other Human CNS Disorders". Current Genomics 10 (3): 154–68. doi:10.2174/138920209788185252. PMC 2705849. PMID 19881909. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2705849.
- ^ Schratt G (December 2009). "microRNAs at the synapse". Nat. Rev. Neurosci. 10 (12): 842–9. doi:10.1038/nrn2763. PMID 19888283.
- ^ Feng J, Sun G, Yan J, Noltner K, Li W, Buzin CH, Longmate J, Heston LL, Rossi J, Sommer SS (2009). Reif, Andreas. ed. "Evidence for X-chromosomal schizophrenia associated with microRNA alterations". PLoS ONE 4 (7): e6121. doi:10.1371/journal.pone.0006121. PMC 2699475. PMID 19568434. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2699475.
- ^ Beveridge NJ, Gardiner E, Carroll AP, Tooney PA, Cairns MJ (September 2009). "Schizophrenia is associated with an increase in cortical microRNA biogenesis". Mol. Psychiatry 15 (12): 1176–89. doi:10.1038/mp.2009.84. PMC 2990188. PMID 19721432. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2990188.
- ^ Pheasant M, Mattick JS (September 2007). "Raising the estimate of functional human sequences". Genome Res. 17 (9): 1245–53. doi:10.1101/gr.6406307. PMID 17690206.
- ^ Bertone P, Stolc V, Royce TE, Rozowsky JS, Urban AE, Zhu X, Rinn JL, Tongprasit W, Samanta M, Weissman S, Gerstein M, Snyder M (December 2004). "Global identification of human transcribed sequences with genome tiling arrays". Science 306 (5705): 2242–6. doi:10.1126/science.1103388. PMID 15539566.
- ^ Ota T, Suzuki Y, Nishikawa T, et al. (January 2004). "Complete sequencing and characterization of 21,243 full-length human cDNAs". Nat. Genet. 36 (1): 40–5. doi:10.1038/ng1285. PMID 14702039.
- ^ Kuhn DE, Martin MM, Feldman DS, Terry AV, Nuovo GJ, Elton TS (January 2008). "Experimental validation of miRNA targets". Methods 44 (1): 47–54. doi:10.1016/j.ymeth.2007.09.005. PMC 2237914. PMID 18158132. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2237914.
- ^ Hüttenhofer A, Schattner P, Polacek N (May 2005). "Non-coding RNAs: hope or hype?". Trends Genet. 21 (5): 289–97. doi:10.1016/j.tig.2005.03.007. PMID 15851066.
Further reading
- This paper discusses the role of microRNAs in viral oncogenesis: Scaria V; Jadhav, Vaibhav (2007). "microRNAs in viral oncogenesis.". Retrovirology 4 (82): 68. doi:10.1186/1742-4690-4-82. PMC 2217556. PMID 18036240. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2217556.
- This paper discusses the role of microRNAs in Host-virus interactions: Scaria V; Hariharan, M; Maiti, S; Pillai, B; Brahmachari, SK (2006). "Host-Virus Interaction: A new role for microRNAs.". Retrovirology 3 (1): 68. doi:10.1186/1742-4690-3-68. PMC 1626483. PMID 17032463. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1626483.
- This paper defines miRNA and proposes guidelines to follow in classifying RNA genes as miRNA: Ambros V, Bartel B, Bartel DP, Burge CB, Carrington JC, Chen X, Dreyfuss G, Eddy SR, Griffiths-Jones S, Marshall M, Matzke M, Ruvkun G, Tuschl T (2003). "A uniform system for microRNA annotation". RNA 9 (3): 277–279. doi:10.1261/rna.2183803. PMC 1370393. PMID 12592000. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1370393.
- This paper discusses the processes that miRNA and siRNAs are involved in, in the context of 2 articles in the same issue of the journal Science: Baulcombe D (2002). "DNA events. An RNA microcosm.". Science 297 (5589): 2002–2003. doi:10.1126/science.1077906. PMID 12242426.
- This paper describes the discovery of lin-4, the first miRNA to be discovered: Lee RC, Feinbaum RL, Ambros V (1993). "The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14". Cell 75 (5): 843–854. doi:10.1016/0092-8674(93)90529-Y. PMID 8252621.
- "This paper showes that the over-expression of a miRNA in mice leads to cancer:" Costinean S, Zanesi N, Pekarsky Y, Tili E, Volinia S, Heerema N, Croce CM (May 2006). "Pre-B cell proliferation and lymphoblastic leukemia/high-grade lymphoma in E(mu)-miR155 transgenic mice". Proc. Natl. Acad. Sci. U.S.A. 103 (18): 7024–9. doi:10.1073/pnas.0602266103. PMC 1459012. PMID 16641092. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1459012.
- This paper discusses the role of microRNAs in skin: Sand M, Gambichler T, Sand D, Skrygan M, Altmeyer P, Bechara FG (2009). "MicroRNAs and the skin: tiny players in the body's largest organ.". J Dermatol Sci 53 (3): 169–175. PMID 19058951. http://www.laboratoriosilesia.com/upfiles/sibi/d_014_micrornas.pdf.
External links
- The miRBase database
- The miRNA Blog
- miR2Disease, a manually curated database documenting known relationships between miRNA dysregulation and human disease.
Protein synthesis Messenger RNA · Ribosomal RNA · Signal recognition particle RNA · Transfer RNA · Transfer-messenger RNARNA processing Gene regulation Antisense RNA · Cis-natural antisense transcript · CRISPR RNA · Long noncoding RNA · MicroRNA · Piwi-interacting RNA · Repeat-associated siRNA · Small interfering RNA · Small temporal RNA · Trans-acting siRNACis-regulatory elements Parasites Other Types of nucleic acids Constituents Nucleobases · Nucleosides · Nucleotides · DeoxynucleotidesRibonucleic acids
(coding and non-coding)translation: mRNA (pre-mRNA/hnRNA) · tRNA · rRNA · tmRNA
regulatory: miRNA · siRNA · piRNA · aRNA · RNAi ·
RNA processing: snRNA · snoRNA
other/ungrouped: gRNA · shRNA · stRNA · ta-siRNADeoxyribonucleic acids Nucleic acid analogues Cloning vectors biochemical families: prot · nucl · carb (glpr, alco, glys) · lipd (fata/i, phld, strd, gllp, eico) · amac/i · ncbs/i · ttpy/i
Wikimedia Foundation. 2010.

