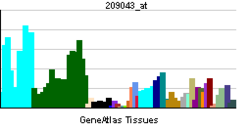
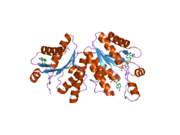
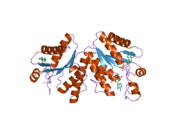
- PAPSS1
-
Bifunctional 3'-phosphoadenosine 5'-phosphosulfate synthetase 1 is an enzyme that in humans is encoded by the PAPSS1 gene.[1][2][3]
Three-prime-phosphoadenosine 5-prime-phosphosulfate (PAPS) is the sulfate donor cosubstrate for all sulfotransferase (SULT) enzymes (Xu et al., 2000). SULTs catalyze the sulfate conjugation of many endogenous and exogenous compounds, including drugs and other xenobiotics. In humans, PAPS is synthesized from adenosine 5-prime triphosphate (ATP) and inorganic sulfate by 2 isoforms, PAPSS1 and PAPSS2 (MIM 603005).[supplied by OMIM][3]
References
- ^ Girard JP, Baekkevold ES, Amalric F (May 1998). "Sulfation in high endothelial venules: cloning and expression of the human PAPS synthetase". FASEB J 12 (7): 603–12. PMID 9576487.
- ^ ul Haque MF, King LM, Krakow D, Cantor RM, Rusiniak ME, Swank RT, Superti-Furga A, Haque S, Abbas H, Ahmad W, Ahmad M, Cohn DH (Oct 1998). "Mutations in orthologous genes in human spondyloepimetaphyseal dysplasia and the brachymorphic mouse". Nat Genet 20 (2): 157–62. doi:10.1038/2458. PMID 9771708.
- ^ a b "Entrez Gene: PAPSS1 3'-phosphoadenosine 5'-phosphosulfate synthase 1". http://www.ncbi.nlm.nih.gov/sites/entrez?Db=gene&Cmd=ShowDetailView&TermToSearch=9061.
Further reading
- Venkatachalam KV (2004). "Human 3'-phosphoadenosine 5'-phosphosulfate (PAPS) synthase: biochemistry, molecular biology and genetic deficiency.". IUBMB Life 55 (1): 1–11. doi:10.1080/1521654031000072148. PMID 12716056.
- Yanagisawa K, Sakakibara Y, Suiko M, et al. (1998). "cDNA cloning, expression, and characterization of the human bifunctional ATP sulfurylase/adenosine 5'-phosphosulfate kinase enzyme.". Biosci. Biotechnol. Biochem. 62 (5): 1037–40. doi:10.1271/bbb.62.1037. PMID 9648242.
- Venkatachalam KV, Akita H, Strott CA (1998). "Molecular cloning, expression, and characterization of human bifunctional 3'-phosphoadenosine 5'-phosphosulfate synthase and its functional domains.". J. Biol. Chem. 273 (30): 19311–20. doi:10.1074/jbc.273.30.19311. PMID 9668121.
- Kurima K, Warman ML, Krishnan S, et al. (1998). "A member of a family of sulfate-activating enzymes causes murine brachymorphism.". Proc. Natl. Acad. Sci. U.S.A. 95 (15): 8681–5. doi:10.1073/pnas.95.15.8681. PMC 21136. PMID 9671738. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=21136.
- Venkatachalam KV, Fuda H, Koonin EV, Strott CA (1999). "Site-selected mutagenesis of a conserved nucleotide binding HXGH motif located in the ATP sulfurylase domain of human bifunctional 3'-phosphoadenosine 5'-phosphosulfate synthase.". J. Biol. Chem. 274 (5): 2601–4. doi:10.1074/jbc.274.5.2601. PMID 9915785.
- Besset S, Vincourt JB, Amalric F, Girard JP (2000). "Nuclear localization of PAPS synthetase 1: a sulfate activation pathway in the nucleus of eukaryotic cells.". FASEB J. 14 (2): 345–54. PMID 10657990.
- Xu ZH, Otterness DM, Freimuth RR, et al. (2000). "Human 3'-phosphoadenosine 5'-phosphosulfate synthetase 1 (PAPSS1) and PAPSS2: gene cloning, characterization and chromosomal localization.". Biochem. Biophys. Res. Commun. 268 (2): 437–44. doi:10.1006/bbrc.2000.2123. PMID 10679223.
- Fuda H, Shimizu C, Lee YC, et al. (2002). "Characterization and expression of human bifunctional 3'-phosphoadenosine 5'-phosphosulphate synthase isoforms.". Biochem. J. 365 (Pt 2): 497–504. doi:10.1042/BJ20020044. PMC 1222679. PMID 11931637. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1222679.
- Strausberg RL, Feingold EA, Grouse LH, et al. (2003). "Generation and initial analysis of more than 15,000 full-length human and mouse cDNA sequences.". Proc. Natl. Acad. Sci. U.S.A. 99 (26): 16899–903. doi:10.1073/pnas.242603899. PMC 139241. PMID 12477932. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=139241.
- Harjes S, Scheidig A, Bayer P (2004). "Expression, purification and crystallization of human 3'-phosphoadenosine-5'-phosphosulfate synthetase 1.". Acta Crystallogr. D Biol. Crystallogr. 60 (Pt 2): 350–2. doi:10.1107/S0907444903027628. PMID 14747722.
- Lansdon EB, Fisher AJ, Segel IH (2004). "Human 3'-phosphoadenosine 5'-phosphosulfate synthetase (isoform 1, brain): kinetic properties of the adenosine triphosphate sulfurylase and adenosine 5'-phosphosulfate kinase domains.". Biochemistry 43 (14): 4356–65. doi:10.1021/bi049827m. PMID 15065880.
- Gerhard DS, Wagner L, Feingold EA, et al. (2004). "The status, quality, and expansion of the NIH full-length cDNA project: the Mammalian Gene Collection (MGC).". Genome Res. 14 (10B): 2121–7. doi:10.1101/gr.2596504. PMC 528928. PMID 15489334. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=528928.
- Harjes S, Bayer P, Scheidig AJ (2005). "The crystal structure of human PAPS synthetase 1 reveals asymmetry in substrate binding.". J. Mol. Biol. 347 (3): 623–35. doi:10.1016/j.jmb.2005.01.005. PMID 15755455.
- Stelzl U, Worm U, Lalowski M, et al. (2005). "A human protein-protein interaction network: a resource for annotating the proteome.". Cell 122 (6): 957–68. doi:10.1016/j.cell.2005.08.029. PMID 16169070.
- Kimura K, Wakamatsu A, Suzuki Y, et al. (2006). "Diversification of transcriptional modulation: large-scale identification and characterization of putative alternative promoters of human genes.". Genome Res. 16 (1): 55–65. doi:10.1101/gr.4039406. PMC 1356129. PMID 16344560. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1356129.
- Sekulic N, Konrad M, Lavie A (2007). "Structural mechanism for substrate inhibition of the adenosine 5'-phosphosulfate kinase domain of human 3'-phosphoadenosine 5'-phosphosulfate synthetase 1 and its ramifications for enzyme regulation.". J. Biol. Chem. 282 (30): 22112–21. doi:10.1074/jbc.M701713200. PMID 17540769.
PDB gallery 1x6v: The crystal structure of human 3'-phosphoadenosine-5'-phosphosulfate synthetase 11xjq: ADP Complex OF HUMAN PAPS SYNTHETASE 11xnj: APS complex of human PAPS synthetase 12ofw: Crystal structure of the APSK domain of human PAPSS1 complexed with 2 APS molecules2ofx: crystal structure of the APSK domain of human PAPSS1 in complex with ADPMg and PAPS2pey: Crystal strucutre of deletion mutant of APS-kinase domain of human PAPS-synthetase 12pez: Crystal structrue of deletion mutant of APS-kinase domain of human PAPS-synthetase 1 in complex with cyclic PAPS and dADPTransferases: phosphorus-containing groups (EC 2.7) 2.7.1-2.7.4:
phosphotransferase/kinase
(PO4)Hexo- · Gluco- · Fructo- (Hepatic) · Galacto- · Phosphofructo- (1, Liver, Muscle, Platelet, 2) · Riboflavin · Shikimate · Thymidine (ADP-thymidine) · NAD+ · Glycerol · Pantothenate · Mevalonate · Pyruvate · Deoxycytidine · PFP · Diacylglycerol · Phosphoinositide 3 (Class I PI 3, Class II PI 3) · Sphingosine · Glucose-1,6-bisphosphate synthase2.7.2: COOH acceptor2.7.6: diphosphotransferase
(P2O7)2.7.7: nucleotidyltransferase
(PO4-nucleoside)DNA-directed DNA polymerase: DNA polymerase I · DNA polymerase II · DNA polymerase III holoenzyme
DNA nucleotidylexotransferase/Terminal deoxynucleotidyl transferase
RNA-directed DNA polymerase: Reverse transcriptase (Telomerase)RNA nucleotidyltransferaseRNA polymerase/DNA-directed RNA polymerase: RNA polymerase I · RNA polymerase II · RNA polymerase III · RNA polymerase IV · Primase · RNA-dependent RNA polymerase
PNPaseUridylyltransferaseGlucose-1-phosphate uridylyltransferase · Galactose-1-phosphate uridylyltransferaseGuanylyltransferasemRNA capping enzymeOther2.7.8: miscellaneous PhosphatidyltransferasesCDP-diacylglycerol—glycerol-3-phosphate 3-phosphatidyltransferase · CDP-diacylglycerol—serine O-phosphatidyltransferase · CDP-diacylglycerol—inositol 3-phosphatidyltransferase · CDP-diacylglycerol—choline O-phosphatidyltransferaseGlycosyl-1-phosphotransferase2.7.10-2.7.13: protein kinase
(PO4; protein acceptor)see tyrosine kinasessee serine/threonine-specific protein kinases2.7.12: protein-dual-specificitysee serine/threonine-specific protein kinases2.7.13: protein-histidineB enzm: 1.1/2/3/4/5/6/7/8/10/11/13/14/15-18, 2.1/2/3/4/5/6/7/8, 2.7.10, 2.7.11-12, 3.1/2/3/4/5/6/7, 3.1.3.48, 3.4.21/22/23/24, 4.1/2/3/4/5/6, 5.1/2/3/4/99, 6.1-3/4/5-6 glycosaminoglycan anabolism glycosaminoglycan catabolism Heparan sulfamidase · N-acetyltransferase · Alpha-N-acetylglucosaminidase · Glucuronidase · N-acetylglucosamine-6-sulfataseCategories:- Human proteins
- Chromosome 4 gene stubs
Wikimedia Foundation. 2010.