- Deoxycytidine kinase
-
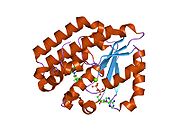
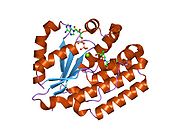
Deoxycytidine kinase, also known as DCK, is an enzyme which in humans is encoded by the DCK gene.[1]
DCK transfers phosphate to deoxycytidine.
Contents
Function
Deoxycytidine kinase (DCK) is required for the phosphorylation of several deoxyribonucleosides and their nucleoside analogs. Deficiency of DCK is associated with resistance to antiviral and anticancer chemotherapeutic agents. Conversely, increased deoxycytidine kinase activity is associated with increased activation of these compounds to cytotoxic nucleoside triphosphate derivatives. DCK is clinically important because of its relationship to drug resistance and sensitivity.[1]
See also
References
Further reading
- Hazra S, Szewczak A, Ort S, Konrad M, Lavie A (February 2011). "Post-translational phosphorylation of serine 74 of human deoxycytidine kinase favors the enzyme adopting the open conformation making it competent for nucleoside binding and release". Biochemistry 50 (14): 2870–80. doi:10.1021/bi2001032. PMC 3071448. PMID 21351740. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=3071448.
- Hazra S, Konrad M, Lavie A (August 2010). "The sugar ring of the nucleoside is required for productive substrate positioning in the active site of human deoxycytidine kinase (dCK): implications for the development of dCK-activated acyclic guanine analogs". J Med Chem 53 (15): 5792–800. doi:10.1021/jm1005379. PMC 2936711. PMID 20684612. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2936711.
- Hazra S, Ort S, Konrad M, Lavie A (August 2010). "Structural and kinetic characterization of human deoxycytidine kinase variants able to phosphorylate 5-substituted deoxycytidine and thymidine analogs". Biochemistry 49 (31): 6748–90. doi:10.1021/bi100839e. PMC 2925221. PMID 20614893. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2925221.
- Hazra S, Sabini E, Ort S, Konrad M, Lavie A (January 2009). "Extending thymidine kinase activity to the catalytic repertoire of human deoxycytidine kinase". Biochemistry 48 (6): 1256–63. doi:10.1021/bi802062w. PMC 2701478. PMID 19159229. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2701478.
- Sabini E, Hazra S, Konrad M, Lavie A (July 2008). "Elucidation of Different Binding Modes of Purine Nucleosides to Human Deoxycytidine Kinase". J. Med. Chem. 51 (14): 4219–25. doi:10.1021/jm800134t. PMC 2636677. PMID 18570408. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2636677.
- Sabini E, Hazra S, Ort S, Konrad M, Lavie A (May 2008). "Structural basis for substrate promiscuity of dCK". J. Mol. Biol. 378 (3): 607–21. doi:10.1016/j.jmb.2008.02.061. PMC 2426910. PMID 18377927. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2426910.
- McSorley T, Ort S, Hazra S, Lavie A, Konrad M (March 2008). "Mimicking phosphorylation of Ser-74 on human deoxycytidine kinase selectively increases catalytic activity for dC and dC analogues". FEBS Lett. 582 (5): 720–4. doi:10.1016/j.febslet.2008.01.048. PMC 2636680. PMID 18258203. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2636680.
- Sabini E, Hazra S, Konrad M, Lavie A (June 2007). "The non-enantioselectivity property of human deoxycytidine kinase explained by structures of the enzyme in complex with l- and d-nucleosides". J. Med. Chem. 50 (13): 3004–14. doi:10.1021/jm0700215. PMC 2586175. PMID 17530837. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2586175.
- Sabini E, Hazra S, Konrad M, Burley SK, Lavie A (2007). "Structural basis for activation of the therapeutic l-nucleoside analogs 3TC and troxacitabine by human deoxycytidine kinase". Nucleic Acids Res. 35 (1): 186–92. doi:10.1093/nar/gkl1038. PMC 1802566. PMID 17158155. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1802566.
- Arnér ES, Eriksson S (1996). "Mammalian deoxyribonucleoside kinases". Pharmacol. Ther. 67 (2): 155–86. doi:10.1016/0163-7258(95)00015-9. PMID 7494863.
- Chottiner EG, Shewach DS, Datta NS, et al. (1991). "Cloning and expression of human deoxycytidine kinase cDNA". Proc. Natl. Acad. Sci. U.S.A. 88 (4): 1531–5. doi:10.1073/pnas.88.4.1531. PMC 51053. PMID 1996353. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=51053.
- Eriksson S, Cederlund E, Bergman T, et al. (1991). "Characterization of human deoxycytidine kinase. Correlation with cDNA sequences". FEBS Lett. 280 (2): 363–6. doi:10.1016/0014-5793(91)80332-W. PMID 2013338.
- Yamada Y, Goto H, Ogasawara N (1984). "Purine nucleoside kinases in human T- and B-lymphoblasts". Biochim. Biophys. Acta 761 (1): 34–40. PMID 6315069.
- Hurley MC, Palella TD, Fox IH (1984). "Human placental deoxyadenosine and deoxyguanosine phosphorylating activity". J. Biol. Chem. 258 (24): 15021–7. PMID 6317685.
- Spasokoukotskaja T, Arnér ES, Brosjö O, et al. (1995). "Expression of deoxycytidine kinase and phosphorylation of 2-chlorodeoxyadenosine in human normal and tumour cells and tissues". Eur. J. Cancer 31A (2): 202–8. doi:10.1016/0959-8049(94)00435-8. PMID 7718326.
- Stegmann AP, Honders MW, Bolk MW, et al. (1993). "Assignment of the human deoxycytidine kinase (DCK) gene to chromosome 4 band q13.3-q21.1". Genomics 17 (2): 528–9. doi:10.1006/geno.1993.1365. PMID 8406512.
- Song JJ, Walker S, Chen E, et al. (1993). "Genomic structure and chromosomal localization of the human deoxycytidine kinase gene". Proc. Natl. Acad. Sci. U.S.A. 90 (2): 431–4. doi:10.1073/pnas.90.2.431. PMC 45676. PMID 8421671. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=45676.
- Johansson M, Brismar S, Karlsson A (1997). "Human deoxycytidine kinase is located in the cell nucleus". Proc. Natl. Acad. Sci. U.S.A. 94 (22): 11941–5. doi:10.1073/pnas.94.22.11941. PMC 23663. PMID 9342341. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=23663.
- Hatzis P, Al-Madhoon AS, Jüllig M, et al. (1998). "The intracellular localization of deoxycytidine kinase". J. Biol. Chem. 273 (46): 30239–43. doi:10.1074/jbc.273.46.30239. PMID 9804782.
- Saada A, Shaag A, Mandel H, et al. (2001). "Mutant mitochondrial thymidine kinase in mitochondrial DNA depletion myopathy". Nat. Genet. 29 (3): 342–4. doi:10.1038/ng751. PMID 11687801.
- Veuger MJ, Heemskerk MH, Honders MW, et al. (2002). "Functional role of alternatively spliced deoxycytidine kinase in sensitivity to cytarabine of acute myeloid leukemic cells". Blood 99 (4): 1373–80. doi:10.1182/blood.V99.4.1373. PMID 11830489.
- Innoceta A, Galluzzi L, Ruzzo A, et al. (2002). "Molecular basis of 2',3'-dideoxycytidine-induced drug resistance in human cells". Mol. Cell. Biochem. 231 (1–2): 173–7. doi:10.1023/A:1014441209108. PMID 11952160.
- Strausberg RL, Feingold EA, Grouse LH, et al. (2003). "Generation and initial analysis of more than 15,000 full-length human and mouse cDNA sequences". Proc. Natl. Acad. Sci. U.S.A. 99 (26): 16899–903. doi:10.1073/pnas.242603899. PMC 139241. PMID 12477932. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=139241.
- Krawiec K, Kierdaszuk B, Shugar D (2003). "Inorganic tripolyphosphate (PPP(i)) as a phosphate donor for human deoxyribonucleoside kinases". Biochem. Biophys. Res. Commun. 301 (1): 192–7. doi:10.1016/S0006-291X(02)03007-3. PMID 12535661.
- van der Wilt CL, Kroep JR, Loves WJ, et al. (2003). "Expression of deoxycytidine kinase in leukaemic cells compared with solid tumour cell lines, liver metastases and normal liver". Eur. J. Cancer 39 (5): 691–7. doi:10.1016/S0959-8049(02)00813-4. PMID 12628850.
- Ge Y, Jensen TL, Matherly LH, Taub JW (2004). "Physical and functional interactions between USF and Sp1 proteins regulate human deoxycytidine kinase promoter activity". J. Biol. Chem. 278 (50): 49901–10. doi:10.1074/jbc.M305085200. PMID 14514691.
- Usova E, Maltseva T, Földesi A, et al. (2005). "Human deoxycytidine kinase as a deoxyribonucleoside phosphorylase". J. Mol. Biol. 344 (5): 1347–58. doi:10.1016/j.jmb.2004.10.016. PMID 15561147.
- Mani RS, Usova EV, Eriksson S, Cass CE (2005). "Fluorescence studies of substrate binding to human recombinant deoxycytidine kinase". Nucleosides Nucleotides Nucleic Acids 23 (8–9): 1343–6. doi:10.1081/NCN-200027609. PMID 15571255.
PDB gallery 1p5z: Structure of human dCK complexed with cytarabine and ADP-MG1p60: Structure of human dCK complexed with 2'-Deoxycytidine and ADP, Space group C 2 2 211p61: Structure of human dCK complexed with 2'-Deoxycytidine and ADP, P 43 21 2 space group1p62: Structure of human dCK complexed with gemcitabine and ADP-MG2a2z: Crystal Structure of human deoxycytidine kinase in complex with deoxycytidine and uridine diphosphate2a30: Crystal structure of human deoxycytidine kinase in complex with deoxycytidine2a7q: Crystal structure of human dCK complexed with clofarabine and ADP2no9: The structure of deoxycytidine kinase complexed with troxacitabine and ADP.2noa: The structure of deoxycytidine kinase complexed with lamivudine and ADP.External links
Transferases: phosphorus-containing groups (EC 2.7) 2.7.1-2.7.4:
phosphotransferase/kinase
(PO4)Hexo- · Gluco- · Fructo- (Hepatic) · Galacto- · Phosphofructo- (1, Liver, Muscle, Platelet, 2) · Riboflavin · Shikimate · Thymidine (ADP-thymidine) · NAD+ · Glycerol · Pantothenate · Mevalonate · Pyruvate · Deoxycytidine · PFP · Diacylglycerol · Phosphoinositide 3 (Class I PI 3, Class II PI 3) · Sphingosine · Glucose-1,6-bisphosphate synthase2.7.2: COOH acceptor2.7.6: diphosphotransferase
(P2O7)2.7.7: nucleotidyltransferase
(PO4-nucleoside)DNA-directed DNA polymerase: DNA polymerase I · DNA polymerase II · DNA polymerase III holoenzyme
DNA nucleotidylexotransferase/Terminal deoxynucleotidyl transferase
RNA-directed DNA polymerase: Reverse transcriptase (Telomerase)RNA nucleotidyltransferaseRNA polymerase/DNA-directed RNA polymerase: RNA polymerase I · RNA polymerase II · RNA polymerase III · RNA polymerase IV · Primase · RNA-dependent RNA polymerase
PNPaseUridylyltransferaseGlucose-1-phosphate uridylyltransferase · Galactose-1-phosphate uridylyltransferaseGuanylyltransferasemRNA capping enzymeOther2.7.8: miscellaneous PhosphatidyltransferasesCDP-diacylglycerol—glycerol-3-phosphate 3-phosphatidyltransferase · CDP-diacylglycerol—serine O-phosphatidyltransferase · CDP-diacylglycerol—inositol 3-phosphatidyltransferase · CDP-diacylglycerol—choline O-phosphatidyltransferaseGlycosyl-1-phosphotransferase2.7.10-2.7.13: protein kinase
(PO4; protein acceptor)see tyrosine kinasessee serine/threonine-specific protein kinases2.7.12: protein-dual-specificitysee serine/threonine-specific protein kinases2.7.13: protein-histidineThis transferase article is a stub. You can help Wikipedia by expanding it.