- Citrinin
-
Citrinin[1] 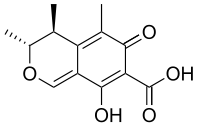 (3R,4S)-8-hydroxy-3,4,5-trimethyl-6-oxo-4,6-dihydro-3H-isochromene-7-carboxylic acidOther namesAntimycin
(3R,4S)-8-hydroxy-3,4,5-trimethyl-6-oxo-4,6-dihydro-3H-isochromene-7-carboxylic acidOther namesAntimycinIdentifiers CAS number 518-75-2 
UNII 3S697X6SNZ 
KEGG C16765 
ChEMBL CHEMBL510139 
Jmol-3D images Image 1 - O=C2C(C(O)=O)=C(O)C1=CO[C@H](C)[C@@H](C)C1=C2C
Properties Molecular formula C13H14O5 Molar mass 250.25 g mol−1 Appearance Lemon-yellow needles Melting point 175 °C (decomp.)
Solubility in water Insoluble  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Citrinin is a mycotoxin originally isolated from Penicillium citrinum. It has since been found to be produced by a variety of other fungi which are used in the production of human foods such as grain, cheese, sake and red pigments. Citrinin has also been found in commercial red yeast rice supplements.[2]
Contents
Toxicity
Citrinin acts as a nephrotoxin in all species in which it has been tested, but its acute toxicity varies.[3] It causes mycotoxic nephropathy in livestock and has been implicated as a cause of Balkan nephropathy and yellow rice fever in humans.
Citrinin is used as a reagent in biological research. It induces mitochondrial permeability pore opening and inhibits respiration by interfering with complex I of the respiratory chain.
Citrinin producers
Citrinin is produced by a variety of fungi including:
- Aspergillus niveus
- Aspergillus ochraceus
- Aspergillus oryzae
- Aspergillus terreus
- Monascus ruber
- Monascus purpureus
- Penicillium citrinum[4]
- Penicillium camemberti
Notes
- ^ Merck Index, 11th Edition, 2329.
- ^ Gordon, R. Y.; Cooperman, T.; Obermeyer, W.; Becker, D. J. (2010). "Marked Variability of Monacolin Levels in Commercial Red Yeast Rice Products: Buyer Beware!". Archives of Internal Medicine 170 (19): 1722–1727. doi:10.1001/archinternmed.2010.382. PMID 20975018.
- ^ Bennett, J. W.; Klich, M. (2003). "Mycotoxins.". Clinical Microbiology Reviews 16 (3): 497–516. doi:10.1128/CMR.16.3.497-516.2003. PMC 164220. PMID 12857779. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=164220.
- ^ citrinin product page from Fermentek
References
- E.J. Da Lozzo et al. J. Biochem. Mol. Toxicol. 1998 12 291
- G.M. Chagas et al. J. Appl. Toxicol. 1995 15 91
- D.Heperkan,B.E.Meriç,G.Şişmanoğlu,G.Dalkılıç,F.Güler. In: Advances in Experimental Medicine & Biology. Edited by A.D. Hocking; J.I.Pitt, R.A.Samson & U.Thrane.2006 571
- Ö.Tokuşoğlu,H.Alpas,F.Bozoğlu. Effect of High Hydrostatic Pressure (HHP) on Mycotoxin Citrinin, Major Phenolics Oleuropein, Hydroxytyrosol, and Total Antioxidant Activity in Black Table Olives. 135-26 Technical Research Paper. 2008 IFT Annual Meeting+Food Expo. June 28-July 1, New Orleans, LA, USA.2008 183
Categories:- Mycotoxins
- Carboxylic acids
- Isochromenes
- Ketones
- Enols
Wikimedia Foundation. 2010.
