- Zearalenone
-
Zearalenone 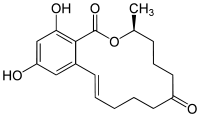 (3S,11E)-14,16-dihydroxy-3-methyl-3,4,5,6,9,10-hexahydro-1H-2-benzoxacyclotetradecine-1,7(8H)-dioneOther namesMycotoxin F2
(3S,11E)-14,16-dihydroxy-3-methyl-3,4,5,6,9,10-hexahydro-1H-2-benzoxacyclotetradecine-1,7(8H)-dioneOther namesMycotoxin F2Identifiers CAS number 17924-92-4 
PubChem 5281576 ChemSpider 4444897 
KEGG C09981 
ChEBI CHEBI:10106 
ChEMBL CHEMBL454173 
Jmol-3D images Image 1 - C[C@H]1CCCC(=O)CCC/C=C/c2cc(cc(c2C(=O)O1)O)O
- InChI=1S/C18H22O5/c1-12-6-5-9-14(19)8-4-2-3-7-13-10-15(20)11-16(21)17(13)18(22)23-12/h3,7,10-12,20-21H,2,4-6,8-9H2,1H3/b7-3+/t12-/m0/s1

Key: MBMQEIFVQACCCH-QBODLPLBSA-N
InChI=1/C18H22O5/c1-12-6-5-9-14(19)8-4-2-3-7-13-10-15(20)11-16(21)17(13)18(22)23-12/h3,7,10-12,20-21H,2,4-6,8-9H2,1H3/b7-3+/t12-/m0/s1
Key: MBMQEIFVQACCCH-QBODLPLBBS
Properties Molecular formula C18H22O5 Molar mass 318.36 g mol−1  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Zearalenone (ZEA), also known as RAL and F-2 mycotoxin, is a potent estrogenic metabolite produced by some Gibberella [1] species.
Several Fusarium species produce toxic substances of considerable concern to livestock and poultry producers: namely, deoxynivalenol, T-2 toxin, HT-2 toxin, diacetoxyscirpenol (DAS) and zearalenone
Zearalenone is the primary toxin causing infertility, abortion or other breeding problems, especially in swine.
Zearalenone is heat-stable and is found worldwide in a number of cereal crops, such as maize, barley, oats, wheat, rice, and sorghum[2] and also in bread.
Contents
Chemical and physical properties
Zearalenone is a white crystalline solid. It exhibits blue-green fluorescence when excited by long wavelength UV light (360) and a more intense green fluorescence when excited with short wavelength UV light (260 nm). In methanol, UV absorption maxima occur at 236 (e =29,700), 274 (e =13,909) and 316 (e =6,020). Maximum fluorescence in ethanol occurs with irradiation at 314nm and with emission at 450nm. Solubility in water is about 0.002g/100ml. It is slightly soluble in hexane and progressively more so in benzene, acetonitrile, methylene chloride, methanol, ethanol, and acetone. It is also soluble in aqueous alkali.
Sampling and analysis
In common with other mycotoxins sampling food commodities for zearalenone must be carried out to obtain samples representative of the consignment under test. Commonly used extraction solvents are aqueous mixtures of methanol, acetonitrile, or ethyl acetate followed by a range of different clean-up procedures that depend in part on the food and on the detection method in use. TLC methods and HPLC are commonly used. HPLC alone is not sufficient as it may often yield false positive results. Today HPLC-MSMS analysis is used to quantify and confirm the presence of zearalenone.
The TLC method for zearalenone is: normal phase silica gel plates, the eluent: 90% dichloromethane, 10% v/v acetone; or reverse phase C18 silica plates; the eluent: 90% v/v methanol, 10% water. Zearalenone gives unmistakable blue luminiscence under UV.[3]
See also
- Zeranol
References
- ^ Zearalenone product page, Fermentek.
- ^ Kuiper-Goodman et al., 1987; Tanaka et al., 1988a.
- ^ Zearalenone, fermentek.co.il.
External links
- Detailed information about mycotoxins
- INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY review on Zearalenone
- MSDS Zearalenone safety data sheet
Categories:- Mycotoxins
- Natural resorcinols
Wikimedia Foundation. 2010.
