- Laminin, alpha 2
-
Laminin, alpha 2 Identifiers Symbols LAMA2; LAMM External IDs OMIM: 156225 MGI: 99912 HomoloGene: 37306 GeneCards: LAMA2 Gene Gene Ontology Molecular function • receptor binding
• structural molecule activityCellular component • extracellular region
• basement membrane
• basal lamina
• laminin-1 complex
• sarcolemmaBiological process • muscle organ development
• regulation of cell adhesion
• regulation of cell migration
• positive regulation of synaptic transmission, cholinergic
• regulation of embryonic developmentSources: Amigo / QuickGO RNA expression pattern 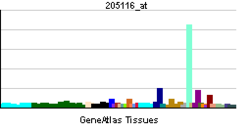
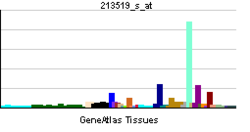
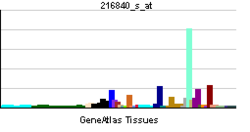
More reference expression data Orthologs Species Human Mouse Entrez 3908 16773 Ensembl ENSG00000196569 ENSMUSG00000019899 UniProt P24043 Q8C9J2 RefSeq (mRNA) NM_000426.3 NM_008481.2 RefSeq (protein) NP_000417.2 NP_032507.2 Location (UCSC) Chr 6:
129.2 – 129.84 MbChr 10:
26.7 – 27.34 MbPubMed search [1] [2] Laminin subunit alpha-2 is a protein that in humans is encoded by the LAMA2 gene.[1][2][3]
Contents
Function
Laminin, an extracellular protein, is a major component of the basement membrane. It is thought to mediate the attachment, migration, and organization of cells into tissues during embryonic development by interacting with other extracellular matrix components. It is composed of three subunits, alpha, beta, and gamma, which are bound to each other by disulfide bonds into a cross-shaped molecule. This gene encodes the alpha 2 chain, which constitutes one of the subunits of laminin 2 (merosin) and laminin 4 (s-merosin). Mutations in this gene have been identified as the cause of congenital merosin-deficient muscular dystrophy. Two transcript variants encoding different proteins have been found for this gene.[3]
References
- ^ Ehrig K, Leivo I, Argraves WS, Ruoslahti E, Engvall E (Jun 1990). "Merosin, a tissue-specific basement membrane protein, is a laminin-like protein". Proc Natl Acad Sci U S A 87 (9): 3264–8. doi:10.1073/pnas.87.9.3264. PMC 53880. PMID 2185464. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=53880.
- ^ Vuolteenaho R, Nissinen M, Sainio K, Byers M, Eddy R, Hirvonen H, Shows TB, Sariola H, Engvall E, Tryggvason K (Feb 1994). "Human laminin M chain (merosin): complete primary structure, chromosomal assignment, and expression of the M and A chain in human fetal tissues". J Cell Biol 124 (3): 381–94. doi:10.1083/jcb.124.3.381. PMC 2119934. PMID 8294519. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2119934.
- ^ a b "Entrez Gene: LAMA2 laminin, alpha 2 (merosin, congenital muscular dystrophy)". http://www.ncbi.nlm.nih.gov/sites/entrez?Db=gene&Cmd=ShowDetailView&TermToSearch=3908.
Further reading
- Timpl R (1997). "Macromolecular organization of basement membranes". Curr. Opin. Cell Biol. 8 (5): 618–24. doi:10.1016/S0955-0674(96)80102-5. PMID 8939648.
- Belkin AM, Stepp MA (2000). "Integrins as receptors for laminins". Microsc. Res. Tech. 51 (3): 280–301. doi:10.1002/1097-0029(20001101)51:3<280::AID-JEMT7>3.0.CO;2-O. PMID 11054877.
- Jones KJ, Morgan G, Johnston H, et al. (2002). "The expanding phenotype of laminin α2 chain (merosin) abnormalities: case series and review". J. Med. Genet. 38 (10): 649–57. doi:10.1136/jmg.38.10.649. PMC 1734735. PMID 11584042. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1734735.
- Hori H, Kanamori T, Mizuta T, et al. (1995). "Human laminin M chain: epitope analysis of its monoclonal antibodies by immunoscreening of cDNA clones and tissue expression". J. Biochem. 116 (6): 1212–9. PMID 7535762.
- Helbling-Leclerc A, Zhang X, Topaloglu H, et al. (1995). "Mutations in the laminin alpha 2-chain gene (LAMA2) cause merosin-deficient congenital muscular dystrophy". Nat. Genet. 11 (2): 216–8. doi:10.1038/ng1095-216. PMID 7550355.
- Yamada H, Shimizu T, Tanaka T, et al. (1994). "Dystroglycan is a binding protein of laminin and merosin in peripheral nerve". FEBS Lett. 352 (1): 49–53. doi:10.1016/0014-5793(94)00917-1. PMID 7925941.
- Bonaldo MF, Lennon G, Soares MB (1997). "Normalization and subtraction: two approaches to facilitate gene discovery". Genome Res. 6 (9): 791–806. doi:10.1101/gr.6.9.791. PMID 8889548.
- Zhang X, Vuolteenaho R, Tryggvason K (1996). "Structure of the human laminin alpha2-chain gene (LAMA2), which is affected in congenital muscular dystrophy". J. Biol. Chem. 271 (44): 27664–9. doi:10.1074/jbc.271.44.27664. PMID 8910357.
- Squarzoni S, Villanova M, Sabatelli P, et al. (1997). "Intracellular detection of laminin alpha 2 chain in skin by electron microscopy immunocytochemistry: comparison between normal and laminin alpha 2 chain deficient subjects". Neuromuscul. Disord. 7 (2): 91–8. doi:10.1016/S0960-8966(96)00420-8. PMID 9131649.
- Allamand V, Sunada Y, Salih MA, et al. (1997). "Mild congenital muscular dystrophy in two patients with an internally deleted laminin alpha2-chain". Hum. Mol. Genet. 6 (5): 747–52. doi:10.1093/hmg/6.5.747. PMID 9158149.
- Durkin ME, Loechel F, Mattei MG, et al. (1997). "Tissue-specific expression of the human laminin alpha5-chain, and mapping of the gene to human chromosome 20q13.2-13.3 and to distal mouse chromosome 2 near the locus for the ragged (Ra) mutation". FEBS Lett. 411 (2–3): 296–300. doi:10.1016/S0014-5793(97)00686-8. PMID 9271224.
- Mrowiec T, Melchar C, Górski A (1998). "HIV-protein-mediated alterations in T cell interactions with the extracellular matrix proteins and endothelium". Arch. Immunol. Ther. Exp. (Warsz.) 45 (2–3): 255–9. PMID 9597096.
- Koch M, Olson PF, Albus A, et al. (1999). "Characterization and Expression of the Laminin γ3 Chain: A Novel, Non-Basement Membrane–associated, Laminin Chain". J. Cell Biol. 145 (3): 605–18. doi:10.1083/jcb.145.3.605. PMC 2185082. PMID 10225960. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2185082.
- Kuang W, Xu H, Vilquin JT, Engvall E (2000). "Activation of the lama2 gene in muscle regeneration: abortive regeneration in laminin alpha2-deficiency". Lab. Invest. 79 (12): 1601–13. PMID 10616210.
- Pegoraro E, Fanin M, Trevisan CP, et al. (2000). "A novel laminin alpha2 isoform in severe laminin alpha2 deficient congenital muscular dystrophy". Neurology 55 (8): 1128–34. PMID 11071490.
- McArthur CP, Wang Y, Heruth D, Gustafson S (2001). "Amplification of extracellular matrix and oncogenes in tat-transfected human salivary gland cell lines with expression of laminin, fibronectin, collagens I, III, IV, c-myc and p53". Arch. Oral Biol. 46 (6): 545–55. doi:10.1016/S0003-9969(01)00014-0. PMID 11311202.
External Links
- GeneReviews/NCBI/NIH/UW entry on Congenital Muscular Dystrophy Overview
- LOVD mutation database: LAMA2
Extracellular matrix Fibril formingOtherFACIT: type IX (COL9A1, COL9A2, COL9A3) · type XII (COL12A1) · COL14A1 · COL16A1 · COL19A1 · COL20A1 · COL21A1 · COL22A1
basement membrane: type IV (COL4A1, COL4A2, COL4A3, COL4A4, COL4A5, COL4A6)
multiplexin: COL15A1 · type XVIII (COL18A1, Endostatin)
transmembrane: COL13A1 · COL17A1 · COL23A1 · COL25A1
other: type VI (COL6A1, COL6A2, COL6A3) · type VII (COL7A1) · type VIII (COL8A1, COL8A2) · type X (COL10A1) · type XI (COL11A1, COL11A2) · COL27A1 · COL28A1 · COL29A1OtherALCAM · Elastin (Tropoelastin) · Vitronectin · FRAS1 · FREM2 · Decorin · FAM20C · ECM1 · Matrix gla protein · Tectorin (TECTA, TECTB)Other see also diseases
B proteins: BY STRUCTURE: membrane, globular (en, ca, an), fibrousCategories:- Human proteins
- Chromosome 6 gene stubs
Wikimedia Foundation. 2010.
