- Systemic scleroderma
-
Systemic sclerosis Classification and external resources 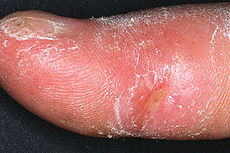
Clinical appearance of acrosclerotic piece-meal necrosis of the thumb in a patient with systemic sclerosis.ICD-10 M34 ICD-9 710.1 OMIM 181750 DiseasesDB 12845 MedlinePlus 000429 eMedicine derm/677 ped/2197 MeSH D012595 Systemic sclerosis or systemic scleroderma[1] is a systemic autoimmune disease or systemic connective tissue disease that is a subtype of scleroderma.[2]
Contents
Signs and symptoms
Skin symptoms
In the skin, systemic sclerosis causes hardening and scarring. The skin may appear tight, reddish or scaly. Blood vessels may also be more visible. Where large areas are affected, fat and muscle wastage may weaken limbs and affect appearance. Also, patients report substantial, even severe and recurrent itching of large skin areas, the source of much affliction as the condition worsens. There is much variation in severity between patients, with some having scleroderma of only a limited area of the skin (such as the fingers) and little involvement of the underlying tissue; while others have progressive skin involvement.[3]
Other organs
Diffuse scleroderma can cause musculoskeletal, pulmonary, gastrointestinal, renal and other complications.[4] Patients with larger amounts of cutaneous involvement are more likely to have involvement of the internal tissues and organs. Most patients (over 80%) have vascular symptoms and Raynaud's phenomenon, which leads to attacks of discoloration of the hands and feet in response to cold. Raynaud's normally affects the fingers and toes. Systemic scleroderma and Raynaud's can cause painful ulcers on the fingers or toes which are known as digital ulcers. Calcinosis (deposition of calcium in lumps under the skin) is also common in systemic scleroderma, and is often seen near the elbows, knees or other joints.
- Musculoskeletal
The first joint symptoms that patients with scleroderma have are typically non specific joint pains, which can lead to arthritis, or cause discomfort in tendons or muscles.[4] Joint mobility, especially of the small joints of the hand, may be restricted by calcinosis or skin thickening.[5] Patients may develop muscle weakness, or myopathy, either from the disease, or its treatments.[6]
- Lungs
Some impairment in lung function is almost universally seen in patients with diffuse scleroderma on pulmonary function testing;[7] however, it does not necessarily cause symptoms, such as shortness of breath. Some patients can develop pulmonary hypertension, or elevation in the pressures of the pulmonary arteries. This can be progressive, and lead to right sided heart failure. The earliest manifestation of this may be a decreased diffusion capacity on pulmonary function testing.
Other pulmonary complications in more advanced disease include aspiration pneumonia, pulmonary hemorrhage and pneumothorax.[4]
- Digestive tract
Diffuse scleroderma can affect any part of the gastrointestinal tract.[8] The most common manifestation in the esophagus is reflux esophagitis, which may be complicated by peptic stricturing, or benign narrowing of the esophagus.[9] This is best initially treated with proton pump inhibitors for acid suppression,[10] but may require bougie dilatation in the case of stricture.[8]
Scleroderma can decrease motility anywhere in the gastrointestinal tract.[8] The most common source of decreased motility involvement is the esophagus and the lower esophageal sphincter, leading to dysphagia and chest pain. As Scleroderma progresses, esophageal involvement from abnormalities in decreased motility may worsen due to progressive fibrosis (scarring). If this is left untreated, acid from the stomach can back up into the esophagus causing esophagitis, and GERD. Further scarring from acid damage to the lower esophagus many times leads to the development of fibrotic narrowing, also known as strictures which can be treated by dilitation, and Barrett's esophagus. The small intestine can also become involved, leading to bacterial overgrowth and malabsorption, of bile salts, fats, carbohydrates, proteins, and vitamins. The colon can be involved, and can cause pseudo-obstruction or ischemic colitis.[4]
Rarer complications include pneumatosis cystoides intestinalis, or gas pockets in the bowel wall, wide mouthed diverticula in the colon and esophagus, and liver fibrosis. Patients with severe gastrointestinal involvement can become profoundly malnourished.[9]
Scleroderma may also be associated with gastric antral vascular ectasia (GAVE), also known as watermelon stomach. This is a condition where atypical blood vessels proliferate usually in a radially symmetric pattern around the pylorus of the stomach. GAVE can be a cause of upper gastrointestinal bleeding or iron deficiency anemia in patients with scleroderma.[9]
- Kidneys
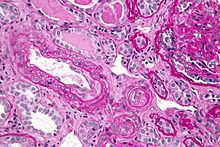 Micrograph showing thrombotic microangiopathy, the histomorphologic finding seen in scleroderma renal crisis. Kidney biopsy. PAS stain.
Micrograph showing thrombotic microangiopathy, the histomorphologic finding seen in scleroderma renal crisis. Kidney biopsy. PAS stain.
Renal involvement, in scleroderma, is considered a poor prognostic factor and frequently a cause of death.[11]
The most important clinical complication of scleroderma involving the kidney is scleroderma renal crisis. Symptoms of scleroderma renal crisis are malignant hypertension (high blood pressure with evidence of acute organ damage), hyperreninemia (high renin levels), azotemia (kidney failure with accumulation of waste products in the blood) and microangiopathic hemolytic anemia (destruction of red blood cells).[12] Apart from the high blood pressure, hematuria (blood in the urine) and proteinuria (protein loss in the urine) may be indicative.[13]
In the past scleroderma renal crisis was almost uniformily fatal.[14] While outcomes have improved significantly with the use of ACE inhibitors[15][16] the prognosis is often guarded, as a significant number of patients are refractory to treatment and develop renal failure. Approximately 5-10% of all scleroderma patients develop renal crisis at some point in the course of their disease.[17] Patients that have rapid skin involvement have the highest risk of renal complications.[17] It is most common in diffuse cutaneous scleroderma, and is often associated with antibodies against RNA polymerase (in 59% of cases). Many proceed to dialysis, although this can be stopped within three years in about a third of cases. Higher age and (paradoxically) a lower blood pressure at presentation make it more likely that dialysis is needed.[18]
Treatments for scleroderma renal crisis include ACE inhibitors, which are also used for prophylaxis,[16][17] and renal transplantation. Transplanted kidneys are known to be affected by scleroderma and patients with early onset renal disease (within one year of the scleroderma diagnosis) are thought to have the highest risk for recurrence.[19]
Diagnosis
Diagnosis is by clinical suspicion, presence of autoantibodies (specifically anti-centromere and anti-scl70/anti-topoisomerase antibodies) and occasionally by biopsy. Of the antibodies, 90% have a detectable anti-nuclear antibody. Anti-centromere antibody is more common in the limited form (80-90%) than in the diffuse form (10%), and anti-scl70 is more common in the diffuse form (30-40%) and in African-American patients (who are more susceptible to the systemic form).[20]
In 1980 the American College of Rheumatology agreed upon diagnostic criteria for scleroderma.[21]
Other conditions may mimic systemic sclerosis by causing hardening of the skin. Diagnostic hints that another disorder is responsible include the absence of Raynaud's phenomenon, a lack of abnormalities in the skin on the hands, a lack of internal organ involvement, and a normal antinuclear antibodies test result.[22]
Causes
There is no clear obvious cause for scleroderma and systemic sclerosis. Genetic predisposition appears to be limited: genetic concordance is small; still, there often is a familial predisposition for autoimmune disease. Polymorphisms in COL1A2 and TGF-β1 may influence severity and development of the disease. There is limited evidence implicating cytomegalovirus (CMV) as the original epitope of the immune reaction. Organic solvents and other chemical agents have been linked with scleroderma.[20]
One of the suspected mechanisms behind the autoimmune phenomenon is the existence of microchimerism, i.e. fetal cells circulating in maternal blood, triggering an immune reaction to what is perceived as "foreign" material.[23][20]
A distinct form of scleroderma and systemic sclerosis may develop in patients with chronic renal failure. This entity, nephrogenic fibrosing dermopathy or nephrogenic systemic fibrosis,[24][25][26][27] has been linked to the exposure to gadolinium-containing radiocontrast.[28]
Bleomycin[29] (a chemotherapeutic agent) and possibly taxane chemotherapy[30] may cause scleroderma, and occupational exposure to solvents has been linked with an increased risk of systemic sclerosis.[31]
Pathophysiology
The overproduction of collagen is thought to result from an autoimmune dysfunction, in which the immune system would start to attack the kinetochore of the chromosomes. This would lead to genetic malfunction of nearby genes. T cells accumulate in the skin; these are thought to secrete cytokines and other proteins that stimulate collagen deposition. Stimulation of the fibroblast, in particular, seems to be crucial to the disease process, and studies have converged on the potential factors that produce this effect.[20]
A significant player in the process is transforming growth factor (TGFβ). This protein appears to be overproduced, and the fibroblast (possibly in response to other stimuli) also overexpresses the receptor for this mediator. An intracellular pathway (consisting of SMAD2/SMAD3, SMAD4 and the inhibitor SMAD7) is responsible for the secondary messenger system that induces transcription of the proteins and enzymes responsible for collagen deposition. Sp1 is a transcription factor most closely studied in this context. Apart from TGFβ, connective tissue growth factor (CTGF) has a possible role.[20] Indeed, a common CTGF gene polymorphism is present at an increased rate in systemic sclerosis.[32]
Damage to endothelium is an early abnormality in the development of scleroderma, and this too seems to be due to collagen accumulation by fibroblasts, although direct alterations by cytokines, platelet adhesion and a type II hypersensitivity reaction have similarly been implicated. Increased endothelin and decreased vasodilation has been documented.[20]
Jimenez & Derk[20] describe three theories about the development of scleroderma:
- The abnormalities are primarily due to a physical agent, and all other changes are secondary or reactive to this direct insult.
- The initial event is fetomaternal cell transfer causing microchimerism, with a second summative cause (e.g. environmental) leading to the actual development of the disease.
- Physical causes lead to phenotypic alterations in susceptible cells (e.g. due to genetic makeup), which then effectuate DNA changes which alter the cell's behavior.
Therapy
There is no cure for scleroderma, though there is treatment for some of the symptoms, including drugs that soften the skin and reduce inflammation. Some patients may benefit from exposure to heat.[33]
Topical/symptomatic
Topical treatment for the skin changes of scleroderma do not alter the disease course, but may improve pain and ulceration. A range of NSAIDs (nonsteroidal anti-inflammatory drugs) can be used to ease painful symptoms, such as naproxen.[citation needed] There is limited benefit from steroids such as prednisone.[citation needed] Episodes of Raynaud's phenomenon sometimes respond to nifedipine or other calcium channel blockers; severe digital ulceration may respond to prostacyclin analogue iloprost, and the dual endothelin-receptor antagonist bosentan may be beneficial for Raynaud's phenomenon.[34] The skin tightness may be treated systemically with methotrexate and ciclosporin.[34]
Kidney disease
Scleroderma renal crisis, the occurrence of acute renal failure and malignant hypertension (very high blood pressure with evidence of organ damage) in people with scleroderma, is effectively treated with drugs from the class of the ACE inhibitors. The benefit of ACE inhibitors extends even to those who have to commence dialysis to treat their kidney disease, and may give sufficient benefit to allow the discontinuation of renal replacement therapy.[34]
Lung disease and pulmonary hypertension
Active alveolitis is often treated with pulses of cyclophosphamide, often together with a small dose of steroids. The benefit of this intervention is modest.[35][36]
Pulmonary hypertension may be treated with epoprostenol, bosentan and possibly aerolized iloprost.[34]
Experimental treatments
Given the difficulty in treating scleroderma, treatments with a smaller evidence base are often tried to control the disease. These include antithymocyte globulin and mycophenolate mofetil; some reports have reported improvements in the skin symptoms as well as delaying the progress of systemic disease, but neither of them have been subjected to large clinical trials.[34]
While still experimental (given its high rate of complications), hematopoietic stem cell transplantation is being studied in patients with severe systemic sclerosis; improvement in life expectancy and severity of skin changes has been noted.[37]
Epidemiology
Systemic scleroderma is a rare disease[38] with and annual incidence of 1 to 2 per 100,000 individuals in the United States. The interval of peak onset starts at age 30[39] to 35[40] and ends at age 50[39] to 55.[40]
In the United States, the prevalence of systemic scleroderma is about 50,000,[40] with different studies giving different estimates, usually ranging between 40,000 and 165,000.[41]
Advocacy
The Juvenile Scleroderma Network is an organization dedicated to provide emotional support and educational information to parents and their children living with juvenile scleroderma, to support pediatric research to identify the cause of and the cure for juvenile sscleroderma, and to enhance public awareness.[42]
In the US, the Scleroderma Research Foundation is dedicated to raise awareness of the disease and assist those who are affected.[43] The Scleroderma Research Foundation sponsors research into the condition.[44] Comedian and television presenter Bob Saget, a board member of the SRF, directed the 1996 ABC TV movie For Hope, starring Dana Delany, which depicts a young woman fatally affected by scleroderma; the film was based on the experiences of Saget's sister Gay.[45]
References
- ^ "systemic sclerosis" at Dorland's Medical Dictionary
- ^ "systemic scleroderma" at Dorland's Medical Dictionary
- ^ Hinchcliff M, Varga J (October 2008). "Systemic sclerosis/scleroderma: a treatable multisystem disease". Am Fam Physician 78 (8): 961–8. PMID 18953973.
- ^ a b c d Klippel, John H.. Primer On the Rheumatic Diseases 11ED. Atlanta, GA: Arthritis Foundation. ISBN 1-912423-16-2.
- ^ Valentini G, Black C (2002). "Systemic sclerosis". Best practice & research. Clinical rheumatology 16 (5): 807–16. doi:10.1053/berh.2002.0258. PMID 12473275.
- ^ Olsen NJ, King LE, Park JH (1996). "Muscle abnormalities in scleroderma". Rheum. Dis. Clin. North Am. 22 (4): 783–96. doi:10.1016/S0889-857X(05)70301-X. PMID 8923596.
- ^ Steen VD (2005). "The lung in systemic sclerosis". Journal of clinical rheumatology 11 (1): 40–6. doi:10.1097/01.rhu.0000152147.38706.db. PMID 16357695.
- ^ a b c Sallam H, McNearney TA, Chen JD (2006). "Systematic review: pathophysiology and management of gastrointestinal dysmotility in systemic sclerosis (scleroderma)". Aliment. Pharmacol. Ther. 23 (6): 691–712. doi:10.1111/j.1365-2036.2006.02804.x. PMID 16556171.
- ^ a b c Rose S, Young MA, Reynolds JC (1998). "Gastrointestinal manifestations of scleroderma". Gastroenterol. Clin. North Am. 27 (3): 563–94. doi:10.1016/S0889-8553(05)70021-2. PMID 9891698.
- ^ Hendel L, Hage E, Hendel J, Stentoft P (1992). "Omeprazole in the long-term treatment of severe gastro-oesophageal reflux disease in patients with systemic sclerosis". Aliment. Pharmacol. Ther. 6 (5): 565–77. doi:10.1111/j.1365-2036.1992.tb00571.x. PMID 1420748.
- ^ Ruangjutipopan S, Kasitanon N, Louthrenoo W, Sukitawut W, Wichainun R (2002). "Causes of death and poor survival prognostic factors in thai patients with systemic sclerosis". Journal of the Medical Association of Thailand 85 (11): 1204–9. PMID 12546318.
- ^ Steen VD, Mayes MD, Merkel PA (2003). "Assessment of kidney involvement". Clin. Exp. Rheumatol. 21 (3 Suppl 29): S29–31. PMID 12889219.
- ^ Steen VD (1994). "Renal involvement in systemic sclerosis". Clin. Dermatol. 12 (2): 253–8. doi:10.1016/S0738-081X(94)90329-8. PMID 8076263.
- ^ Steen VD (2003). "Scleroderma renal crisis". Rheum. Dis. Clin. North Am. 29 (2): 315–33. doi:10.1016/S0889-857X(03)00016-4. PMID 12841297.
- ^ Rhew EY, Barr WG (2004). "Scleroderma renal crisis: new insights and developments". Current rheumatology reports 6 (2): 129–36. doi:10.1007/s11926-004-0057-5. PMID 15016343.
- ^ a b Steen VD, Medsger TA (2000). "Long-term outcomes of scleroderma renal crisis". Ann. Intern. Med. 133 (8): 600–3. PMID 11033587.
- ^ a b c Jimenez S, Koenig AS. Scleroderma. eMedicine.com. Accessed: May 22, 2006.
- ^ Penn H, Howie AJ, Kingdon EJ, et al. (August 2007). "Scleroderma renal crisis: patient characteristics and long-term outcomes". QJM 100 (8): 485–94. doi:10.1093/qjmed/hcm052. PMID 17601770. http://qjmed.oxfordjournals.org/cgi/content/full/100/8/485.
- ^ Pham PT, Pham PC, Danovitch GM, et al. (October 2005). "Predictors and risk factors for recurrent scleroderma renal crisis in the kidney allograft: case report and review of the literature". Am. J. Transplant. 5 (10): 2565–9. doi:10.1111/j.1600-6143.2005.01035.x. PMID 16162209. http://www.blackwell-synergy.com/doi/full/10.1111/j.1600-6143.2005.01035.x.
- ^ a b c d e f g Jimenez SA, Derk CT (2004). "Following the molecular pathways toward an understanding of the pathogenesis of systemic sclerosis". Ann. Intern. Med. 140 (1): 37–50. PMID 14706971.
- ^ "Preliminary criteria for the classification of systemic sclerosis (scleroderma). Subcommittee for scleroderma criteria of the American Rheumatism Association Diagnostic and Therapeutic Criteria Committee". Arthritis Rheum. 23 (5): 581–90. 1980. doi:10.1002/art.1780230510. PMID 7378088. Available online at "1980 Criteria for the Classification of Systemic Sclerosis". http://www.rheumatology.org/publications/classification/systsclr.asp. Retrieved 2007-08-05.
- ^ Moenning R and Grau RG (March 30, 2009). "Skin hardening, but is it systemic sclerosis?". The Journal of Musculoskeletal Medicine. http://jmm.consultantlive.com/display/article/1145622/1391088.
- ^ Bianchi DW (2000). "Fetomaternal cell trafficking: a new cause of disease?". Am. J. Med. Genet. 91 (1): 22–8. doi:10.1002/(SICI)1096-8628(20000306)91:1<22::AID-AJMG4>3.0.CO;2-3. PMID 10751084.
- ^ Abdel-Kader K, Patel PR, Kallen AJ, Sinkowitz-Cochran RL, Bolton WK, Unruh ML (March 2010). "Nephrogenic Systemic Fibrosis: A Survey of Nephrologists' Perceptions and Practices". Clin J Am Soc Nephrol 5 (6): 964–71. doi:10.2215/CJN.00140110. PMC 2879309. PMID 20299369. http://cjasn.asnjournals.org/cgi/pmidlookup?view=long&pmid=20299369.
- ^ "[Nephrogenic systemic fibrosis or kidney failure--how great is the risk?]" (in German). Rofo 182 (2): 114–5. February 2010. PMID 20120045.
- ^ Panos A, Milas F, Kalakonas S, Myers PO (2010). "Cardiac autotransplantation for aortic and mitral valve replacement in a patient with nephrogenic systemic fibrosis". Hellenic J Cardiol 51 (1): 64–6. PMID 20118047. http://www.hellenicjcardiol.org/archive/full_text/2010/1/2010_1_64.pdf.
- ^ Fine DM, Perazella MA (January 2010). "Nephrogenic systemic fibrosis: what the hospitalist needs to know". J Hosp Med 5 (1): 46–50. doi:10.1002/jhm.493. PMID 20063400.
- ^ Boyd AS, Zic JA, Abraham JL (2007). "Gadolinium deposition in nephrogenic fibrosing dermopathy". J. Am. Acad. Dermatol. 56 (1): 27–30. doi:10.1016/j.jaad.2006.10.048. PMID 17109993.
- ^ Sharma SK, Handa R, Sood R, et al. (2004). "Bleomycin-induced scleroderma". The Journal of the Association of Physicians of India 52: 76–7. PMID 15633728.
- ^ Farrant PB, Mortimer PS, Gore M (2004). "Scleroderma and the taxanes. Is there really a link?". Clin. Exp. Dermatol. 29 (4): 360–2. doi:10.1111/j.1365-2230.2004.01519.x. PMID 15245529.
- ^ Kettaneh A, Al Moufti O, Tiev KP, et al. (2007). "Occupational exposure to solvents and gender-related risk of systemic sclerosis: a metaanalysis of case-control studies". J. Rheumatol. 34 (1): 97–103. PMID 17117485.
- ^ Fonseca C, Lindahl GE, Ponticos M, et al. (September 2007). "A polymorphism in the CTGF promoter region associated with systemic sclerosis". N. Engl. J. Med. 357 (12): 1210–20. doi:10.1056/NEJMoa067655. PMID 17881752. http://content.nejm.org/cgi/content/full/357/12/1210.
- ^ Oliver GF, Winkelmann RK (1989). "The current treatment of scleroderma". Drugs 37 (1): 87–96. doi:10.2165/00003495-198937010-00006. PMID 2651089.
- ^ a b c d e Zandman-Goddard G, Tweezer-Zaks N, Shoenfeld Y (2005). "New therapeutic strategies for systemic sclerosis--a critical analysis of the literature". Clin. Dev. Immunol. 12 (3): 165–73. doi:10.1080/17402520500233437. PMC 2275417. PMID 16295521. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2275417. Full text at PMC: 2275417
- ^ Tashkin DP, Elashoff R, Clements PJ, et al. (June 2006). "Cyclophosphamide versus placebo in scleroderma lung disease". N. Engl. J. Med. 354 (25): 2655–66. doi:10.1056/NEJMoa055120. PMID 16790698. http://content.nejm.org/cgi/content/full/354/25/2655.
- ^ Hoyles RK, Ellis RW, Wellsbury J, et al. (December 2006). "A multicenter, prospective, randomized, double-blind, placebo-controlled trial of corticosteroids and intravenous cyclophosphamide followed by oral azathioprine for the treatment of pulmonary fibrosis in scleroderma". Arthritis Rheum. 54 (12): 3962–70. doi:10.1002/art.22204. PMID 17133610. http://www3.interscience.wiley.com/cgi-bin/fulltext/113490260/HTMLSTART.
- ^ Nash RA, McSweeney PA, Crofford LJ, et al. (2007). "High-dose immunosuppressive therapy and autologous hematopoietic cell transplantation for severe systemic sclerosis: long-term follow-up of the U.S. multicenter pilot study". Blood 110 (4): 1388–96. doi:10.1182/blood-2007-02-072389. PMC 1939909. PMID 17452515. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1939909.
- ^ WrongDiagnosis > Diseases » Scleroderma » Prevalence Retrieved on Dec 13, 2009
- ^ a b Systemic sclerosis (scleroderma) and pregnancy By Bonnie L Bermas, MD. Retrieved on Dec 13, 2009
- ^ a b c University of maryland Medical Center > Medical Reference > Patient Education > Scleroderma - Risk Factors Harvey Simon, MD, Reviewed last on: 3/17/2009
- ^ CureResearch > Prevalence and Incidence of Scleroderma Last revision: June 13, 2003
- ^ "Juvenile Scleroderma Network". http://www.jsdn.org/. Retrieved 2008-05-11.
- ^ "Scleroderma Foundation". http://www.scleroderma.org/. Retrieved 2008-05-11.
- ^ "Scleroderma Research Foundation". http://www.srfcure.org. Retrieved 2008-05-11..
- ^ For Hope at the Internet Movie Database
External links
- UK Raynaud's & Scleroderma Association
- DermnetNZ: Systemic sclerosis
- Juvenile Scleroderma Network
- Juvenile Systemic Sclerosis
- Scleroderma Foundation
- Scleroderma Society of Ontario
- International Scleroderma Network
- The Scleroderma Research Foundation
- UK Scleroderma Society
- Scleroderma Information from Johns Hopkins University
- Scleroderma Australia
Systemic CT disorders (M32–M36, 710) General Other hypersensitivity/autoimmune Other Categories:- Mucinoses
- Connective tissue diseases
- Autoimmune diseases
- Diseases involving the fasciae
- Systemic connective tissue disorders
Wikimedia Foundation. 2010.