- Microchimerism
-
Microchimerism is the presence of a small number of cells that originate from another individual and are therefore genetically distinct from the cells of the host individual. This phenomenon may be related to certain types of autoimmune diseases; however, the mechanisms responsible for this relationship are unclear.
Contents
Types of microchimerism
Human
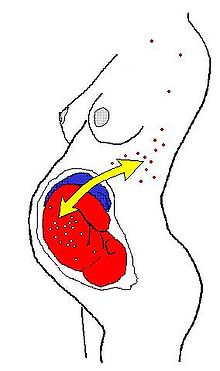
In humans (and perhaps in all Placentals) the most common form is fetomaternal microchimerism (also known as fetal cell microchimerism or fetal chimerism) whereby cells from a fetus pass through the placenta and establish cell lineages within the mother. Fetal cells have been documented to persist and multiply in the mother for several decades.[1][2] The exact phenotype of these cells is unknown, although several different cell types have been identified, such as various immune lineages, mesenchymal stem cells, and placental-derived cells. The potential health consequences of these cells are currently unknown. One hypothesis is that these fetal cells might trigger a graft-versus-host reaction leading to autoimmune disease. This offers a potential explanation for why many autoimmune diseases are more prevalent in middle-aged women.[3] The other main theory is that fetal cells home to injured or diseased maternal tissue where they act as stem cells and participate in repair.[4][5] It is also possible that the fetal cells are merely innocent bystanders and have no effect on maternal health.[6]
After giving birth, about 50-75 % of women carry fetal immune cell lines. Maternal immune cells are also found in the offspring yielding in maternal→fetal microchimerism, though this phenomenon is about half as frequent as the former .[7]
Microchimerism had also been shown to exist after blood transfusions to a severely immunocompromised population of patients who suffered trauma.[8]
Animal
Microchimerism occurs in most pairs of twins in cattle. In cattle (and other bovines), the placentae of fraternal twins usually fuse and the twins share blood circulation, resulting in exchange of cell lines. If the twins are a male-female pair, the male hormones from the bull calf have the effect of partially masculinising the heifer (female), creating a martin heifer or freemartin. Freemartins appear female, but are infertile and so cannot be used for breeding or dairy production. Microchimerism provides a method of diagnosing the condition, because male genetic material can be detected in a blood sample.[9]
Relationship with autoimmune diseases and breast cancer
Microchimerism has been implicated in autoimmune diseases. Independent studies repeatedly suggested that microchimeric cells of fetal origin may be involved in the pathogenesis of systemic sclerosis.[2][10] Moreover, microchimeric cells of maternal origin may be involved in the pathogenesis of a group of autoimmune diseases found in children, i.e. juvenile idiopathic inflammatory myopathies (one example would be juvenile dermatomyositis).[11] Microchimerism has now been further implicated in other autoimmune diseases, including systemic lupus erythematosus.[12] Contrarily, an alternative hypothesis on the role of microchimeric cells in lesions is that they may be facilitating tissue repair of the damaged organ.[13]
Moreover, fetal immune cells have also been frequently found in breast cancer stroma as compared to samples taken from healthy women. It is not clear, however, whether fetal cell lines promote the development of tumors or, contrarily, protect women from developing breast carcinoma.[14][15]
Microchimerism and disease within the context of a tripartite conflict
A recent hypothesis[16] interprets this relationship by considering fetal, maternal, and paternal adaptive interests separately and in interaction with one another. Theoretically, fetuses may benefit from immunological information gathered by their migrant immune cells in the maternal body provided that many of them return into the fetus. They may also benefit from improved maternal defense, provided that fetal cell lines (carrying partly paternal resistance alleles) contribute to maternal defense against pathogens or tumors. However, fetuses may be jeopardized by a selfish maternal usage of fetomaternal microchimerism – i.e. some mothers get pregnant only to improve their immune system and then to abort. The use of microchimeric cells by the maternal immune system may contribute to the adaptive benefits of female choosiness and polyandry. While fathers may enjoy an indirect benefit from enhanced fetal and maternal health, they also face the risk of wasting their sexual efforts due to selfish pregnancies of cheating females. Paternal alleles acting via clones of microchimeric cells in the maternal body could launch an immunological attack against the non-kin sperm in the female genitalia, or against the non-kin fetus in the womb. Thus microchimeric cells carrying partly the alleles of a former father may launch an attack on a new embryo fathered by a new male. Furthermore, an intraspecific version of Zahavi’s Mafia Hypothesis[17] could explain a potential interaction between the abortion of fetuses and a subsequent rise of an autoimmune disease. This hypothesis suggests that males may be capable of provoking microchimerism-induced autoimmune-like diseases in the mother in revenge of selfish pregnancies. This hypothetic paternal threat could increase the maternal costs associated to selfish pregnancies, thus reduce the chance that males just waste their efforts.
According to the traditional medical thinking, autoimmune disease may cause infertility, habitual abortion and miscarriage.[18] However, this interpretation does not explain why autoimmune diseases (at least several types of them) occur mostly in women, and cannot explain why miscarriage tends to precede the rise of autoimmune problems.[19]
On the contrary, considering the tripartite immune conflict among the fetus, the mother and father as realized by means of a two-way traffic of immune cells through the placenta can nicely explain these phenomena.
See also
- Chimerism
- Allotransplantation
References
- ^ Bianchi, DW; Zickwold GK, Weil GJ, Sylvester S, DeMaria MA. (1996). "Male fetal progenitor cells persist in maternal blood for as long as 27 years postpartum". Proc Natl Acad Sci U S A 93: 705–708. doi:10.1073/pnas.93.2.705. PMC 40117. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=40117.
- ^ a b Evans PC, Lambert N, Maloney S, Furst DE, Moore JM, Nelson JL (1999). "Long-term fetal microchimerism in peripheral blood mononuclear cell subsets in healthy women and women with scleroderma". Blood 93 (6): 2033–2037. PMID 10068676.
- ^ Nelson, JL (1996). "Maternal-fetal immunology and autoimmune disease: is some autoimmune disease auto-alloimmune or allo-autoimmune?". Arthritis Rheum 39 (2): 191–194.
- ^ Khosrotehrani, K; Johnson KL, Cha DH, Salomon RN, Bianchi DW (2004). "Transfer of fetal cells with multilineage potential to maternal tissue". Journal of the American Medical Association 292: 75–80.
- ^ Nguyen Huu, S; Oster M, Avril MF, Boitier F, Mortier L, Richard MA, Kerob D, Maubec E, Souteyrand P, Moguelet P, Khosrotehrani K, Aractingi S. (2009). "Fetal microchimeric cells participate in tumour angiogenesis in melanomas occurring during pregnancy". Am J Cardiovasc Pathol 174: 630–637.
- ^ Johnson, KL; Bianchi DW (2004). "Fetal cells in maternal tissue following pregnancy: what are the consequences?". Hum Reprod Update 10 (6): 497–502.
- ^ Loubičre LS, Lambert NC, Flinn LJ, Erickson TD, Yan Z, Guthrie KA, et al. (2006). "Maternal microchimerism in healthy adults in lymphocytes, monocyte/macrophages and NK cells". Lab Invest 86 (11): 1185–92. doi:10.1038/labinvest.3700471. PMID 16969370.
- ^ Reed W, Lee TH, Norris PJ, Utter GH, Busch MP (2007). "Transfusion-associated microchimerism: a new complication of blood transfusions in severely injured patients". Seminars in Hematology 44 (1): 24–31. doi:10.1053/j.seminhematol.2006.09.012. PMID 17198844.
- ^ A. Fujishiro, K. Kawakura, Y-I. Miyake, Y. Kaneda, "A fast, convenient diagnosis of the bovine freemartin syndrome using polymerase chain reaction", Theriogenology, 43 (5), pp 883-891 (1 April 1995)
- ^ Artlett CM, Smith JB, Jimenez SA (1998). "Identification of fetal DNA and cells in skin lesions from women with systemic sclerosis". New England Journal of Medicine 338 (17): 1186–1196. doi:10.1056/NEJM199804233381704. PMID 9554859. http://content.nejm.org/cgi/content/full/338/17/1186.
- ^ Artlett CM, Ramos R, Jimenez SA, Patterson K, Miller FW, Rider LG (2000). "Chimeric cells of maternal origin in juvenile idiopathic inflammatory myopathies. Childhood Myositis Heterogeneity Collaborative Group". Lancet 356 (9248): 2155–2156. doi:10.1016/S0140-6736(00)03499-1. PMID 11191545.
- ^ Johnson KL, McAlindon TE, Mulcahy E, Bianchi DW (2001). "Microchimerism in a female patient with systemic lupus erythematosus". Arthritis & Rheumatism 44: 2107–2111. doi:10.1002/1529-0131(200109)44:9<2107::AID-ART361>3.0.CO;2-9. http://www3.interscience.wiley.com/journal/85512936/abstract?CRETRY=1&SRETRY=0.
- ^ Gilliam AC (2006). "Microchimerism and skin disease: true-true unrelated?". Journal of Investigative Dermatology 126 (2): 239–241. doi:10.1038/sj.jid.5700061. PMID 16418731.
- ^ Gadi VK, Nelson JL (2007). "Fetal microchimerism in women with breast cancer". Cancer Research 67 (19): 9035–9038. doi:10.1158/0008-5472.CAN-06-4209. PMID 17909006. http://cancerres.aacrjournals.org/cgi/content/full/67/19/9035.
- ^ Dubernard G et al. (2008). "Breast cancer stroma frequently recruits fetal derived cells during pregnancy". Breast Cancer Research 10 (1): R14. doi:10.1186/bcr1860. PMC 2374970. PMID 18271969. http://breast-cancer-research.com/content/10/1/R14#B38.
- ^ Apari P, Rózsa L (2009). "The tripartite immune conflict in placentals and a hypothesis on fetal→maternal Microchimerism". Medical Hypotheses 72 (1): 52–54. doi:10.1016/j.mehy.2008.08.021. PMID 18930355. http://www.zoologia.hu/list/microchimerism.pdf.
- ^ Zahavi A (1979). "Parasitism and nest predation in parasitic cuckoos". The American Naturalist 113: 157–159.
- ^ Nelson JL (1998). "Microchimerism and autoimmune disease". New England Journal of Medicine 338 (17): 1224–1225. doi:10.1056/NEJM199804233381711. PMID 9554866.
- ^ Gleicher N (1999). "Reproductive failure prior to the onset of clinical autoimmune disease". Rheumatology 38 (6): 485–487. doi:10.1093/rheumatology/38.6.485. PMID 10402065. http://rheumatology.oxfordjournals.org/cgi/reprint/38/6/485?ijkey=7465addffa328253ddd1a5b19a7df33539d8db42&keytype2=tf_ipsecsha.
Categories:- Autoimmune diseases
- Diseases and disorders
- Reproduction
- Mating
- Evolutionary biology
- Sexual selection
Wikimedia Foundation. 2010.