- Opal
-
For other uses, see Opal (disambiguation).
Opal 
An opal bracelet. The stone size is 18 by 15 mm (0.7 by 0.6 in)General Category Mineraloid Chemical formula Hydrated silica. SiO2·nH2O Identification Color Colorless, white, yellow, red, orange, green, brown, black, blue Crystal habit Irregular veins, in masses, in nodules Crystal system Amorphous[1] Cleavage None[1] Fracture Conchoidal to uneven[1] Mohs scale hardness 5.5–6[1] Luster Subvitreous to waxy[1] Streak White Diaphaneity opaque, translucent, transparent Specific gravity 2.15 (+.08, -.90)[1] Density 2.09 Polish luster Vitreous to resinous[1] Optical properties Single refractive, often anomalous double refractive due to strain[1] Refractive index 1.450 (+.020, -.080) Mexican opal may read as low as 1.37, but typically reads 1.42–1.43[1] Birefringence none[1] Pleochroism None[1] Ultraviolet fluorescence black or white body color: inert to white to moderate light blue, green, or yellow in long and short wave. May also phosphoresce; common opal: inert to strong green or yellowish green in long and short wave, may phosphoresce; fire opal: inert to moderate greenish brown in long and short wave, may phosphoresce.[1] Absorption spectra green stones: 660nm, 470nm cutoff[1] Diagnostic features darkening upon heating Solubility hot saltwater, bases, methanol, humic acid, hydrofluoric acid References [2][3] Opal is an amorphous form of silica related to quartz, a mineraloid form, not a mineral. 3% to 21% of the total weight is water, but the content is usually between 6% to 10%. It is deposited at a relatively low temperature and may occur in the fissures of almost any kind of rock, being most commonly found with limonite, sandstone, rhyolite, marl and basalt. Opal is the national gemstone of Australia, which produces 97% of the world's supply.[4]
Opal's internal structure makes it diffract light; depending on the conditions in which it formed it can take on many colors. Opal ranges from clear through white, gray, red, orange, yellow, green, blue, magenta, rose, pink, slate, olive, brown, and black. Of these hues, the reds against black are the most rare, whereas white and greens are the most common. It varies in optical density from opaque to semi-transparent. For gemstone use, its natural color is often enhanced by placing thin layers of opal on a darker underlying stone, like basalt.
Contents
Precious opal
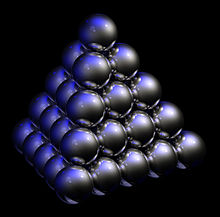
Precious opal shows a variable interplay of internal colors and even though it is a mineraloid, it does have an internal structure. At micro scales precious opal is composed of silica spheres some 150 to 300 nm in diameter in a hexagonal or cubic close-packed lattice. These ordered silica spheres produce the internal colors by causing the interference and diffraction of light passing through the microstructure of the opal.[5] It is the regularity of the sizes and the packing of these spheres that determines the quality of precious opal. Where the distance between the regularly packed planes of spheres is approximately half the wavelength of a component of visible light, the light of that wavelength may be subject to diffraction from the grating created by the stacked planes. The spacing between the planes and the orientation of planes with respect to the incident light determines the colors observed. The process can be described by Bragg's Law of diffraction.
Visible light of diffracted wavelengths cannot pass through large thicknesses of the opal. This is the basis of the optical band gap in a photonic crystal, of which opal is the best known natural example. In addition, microfractures may be filled with secondary silica and form thin lamellae inside the opal during solidification. The term opalescence is commonly and erroneously used to describe this unique and beautiful phenomenon, which is correctly termed play of color. Contrarily, opalescence is correctly applied to the milky, turbid appearance of common or potch opal. Potch does not show a play of color.
The veins of opal displaying the play of color are often quite thin, and this has given rise to unusual methods of preparing the stone as a gem. An opal doublet is a thin layer of opal, backed by a swart mineral such as ironstone, basalt, or obsidian. The darker backing emphasizes the play of color, and results in a more attractive display than a lighter potch.
Combined with modern techniques of polishing, doublet opal produces similar effect of black or boulder opals at a mere fraction of the price. Doublet opal also has the added benefit of having genuine opal as the top visible and touchable layer, unlike triplet opals.
The triplet-cut opal backs the colored material with a dark backing, and then has a domed cap of clear quartz or plastic on top, which takes a high polish and acts as a protective layer for the opal. The top layer also acts as a magnifier, to emphasize the play of color of the opal beneath, which is often of lower quality. Triplet opals therefore have a more artificial appearance, and are not classed as precious opal.
Common opal
Besides the gemstone varieties that show a play of color, there are other kinds of common opal such as the milk opal, milky bluish to greenish (which can sometimes be of gemstone quality); resin opal, which is honey-yellow with a resinous luster; wood opal, which is caused by the replacement of the organic material in wood with opal;[6] menilite, which is brown or grey; hyalite, a colorless glass-clear opal sometimes called Muller's Glass; geyserite, also called siliceous sinter, deposited around hot springs or geysers; and diatomite or diatomaceous earth, the accumulations of diatom shells or tests.
Other varieties of opal
Girasols, more commonly called fire opals, are transparent to translucent opals with warm body colors of yellow, orange, orange-yellow or red. They do not usually show any play of color, although occasionally a stone will exhibit bright green flashes. The most famous source of fire opals is the state of Querétaro in Mexico; these opals are commonly called Mexican fire opals. Fire opals that do not show play of color are sometimes referred to as jelly opals.
Peruvian opal (also called blue opal) is a semi-opaque to opaque blue-green stone found in Peru which is often cut to include the matrix in the more opaque stones. It does not display pleochroism.
Sources of opal
 Polished opal from Yowah (Yowah Nut[7]), Queensland, Australia
Polished opal from Yowah (Yowah Nut[7]), Queensland, Australia
Australia produces around 97% of the world's opal. 90% is called 'light opal' or white and crystal opal. White makes up 60% of the opal productions but cannot be found in all of the opal fields. Crystal opal or pure hydrated silica makes up 30% of the opal produced, 8% is black and only 2% is boulder opal.[citation needed]
The town of Coober Pedy in South Australia is a major source of opal. The world's largest and most valuable gem opal "Olympic Australis" was found in August 1956 at the "Eight Mile" opal field in Coober Pedy. It weighs 17,000 carats (3450 grams) and is 11 inches (280 mm) long, with a height of 43⁄4 inches (120 mm) and a width of 41⁄2 inches (110 mm). It is valued at AUD$2,500,000[8]
Mintabie Opal Fields located approximately 250 km north west of Coober Pedy has also produced large quantities of Crystal opal and also the rarer black opal. Over the years it has been sold overseas incorrectly as Coober Pedy Opal. The black opal is said to be some of the best examples found in Australia.
Andamooka in South Australia is also a major producer of matrix opal, crystal opal, and black opal. Another Australian town, Lightning Ridge in New South Wales, is the main source of black opal, opal containing a predominantly dark background (dark-gray to blue-black displaying the play of color). Boulder opal consists of concretions and fracture fillings in a dark siliceous ironstone matrix. It is found sporadically in western Queensland, from Kynuna in the north, to Yowah and Koroit in the south.[9] The rarest type of Australian opal is "pipe" opal, closely related to boulder opal, which forms in sandstone with some iron-ore content, usually as fossilized tree roots. Its largest quantities are found around Jundah in South West Queensland. Australia also has opalised fossil remains,[10] including dinosaur bones in New South Wales, and marine creatures in South Australia.
The Virgin Valley opal fields of Humboldt County in northern Nevada produce a wide variety of precious black, crystal, white, fire, and lemon opal. The black fire opal is the official gemstone of Nevada. Most of the precious opal is partial wood replacement. Miocene age opalised teeth, bones, fish, and a snake head have been found. Some of the opal has high water content and may desiccate and crack when dried. The largest black opal in the Smithsonian Institution comes from the Royal Peacock opal mine in the Virgin Valley.[citation needed]
Another source of white base opal or creamy opal in the United States is Spencer, Idaho.[citation needed] A high percentage of the opal found there occurs in thin layers.
Other significant deposits of precious opal around the world can be found in the Czech Republic, Slovakia, Hungary, Turkey, Indonesia, Brazil (in Pedro II, Piauí[11]), Honduras, Guatemala, Nicaragua and Ethiopia.
In late 2008, NASA announced that it had discovered opal deposits on Mars.[12]
Synthetic opal
As well as occurring naturally, opals of all varieties have been synthesized experimentally and commercially. The discovery of the ordered sphere structure of precious opal led to its synthesis by Pierre Gilson in 1974.[5] The resulting material is distinguishable from natural opal by its regularity; under magnification, the patches of color are seen to be arranged in a "lizard skin" or "chicken wire" pattern. Furthermore, synthetic opals do not fluoresce under UV light. Synthetics are also generally lower in density and are often highly porous.
Two notable producers of synthetic opal are the companies Kyocera and Inamori of Japan. Most so-called synthetics, however, are more correctly termed "imitation opal", as they contain substances not found in natural opal (e.g., plastic stabilizers). The imitation opals seen in vintage jewelry are often foiled glass, glass-based "Slocum stone", or later plastic materials.
Other research in macroporous structures have yielded highly ordered materials that have similar optical properties to opals and have been used in cosmetics.[13]
Local atomic structure of opals
The lattice of spheres of opal that cause the interference with light are several hundred times larger than the fundamental structure of crystalline silica. As a mineraloid, there is no unit cell that describes the structure of opal. Nevertheless, opals can be roughly divided into those that show no signs of crystalline order (amorphous opal) and those that show signs of the beginning of crystalline order, commonly termed cryptocrystalline or microcrystalline opal.[14] Dehydration experiments and infrared spectroscopy have shown that most of the H2O in the formula of SiO2·nH2O of opals is present in the familiar form of clusters of molecular water. Isolated water molecules, and silanols, structures such as Si-O-H, generally form a lesser proportion of the total and can reside near the surface or in defects inside the opal.
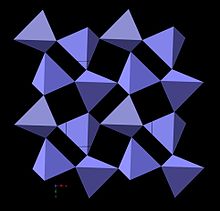
The structure of low-pressure polymorphs of anhydrous silica consist of frameworks of fully corner bonded tetrahedra of SiO4. The higher temperature polymorphs of silica cristobalite and tridymite are frequently the first to crystallize from amorphous anhydrous silica, and the local structures of microcrystalline opals also appear to be closer to that of cristobalite and tridymite than to quartz. The structures of tridymite and cristobalite are closely related and can be described as hexagonal and cubic close-packed layers. It is therefore possible to have intermediate structures in which the layers are not regularly stacked.
Microcrystalline opal
Opal-CT has been interpreted as consisting of clusters of stacking of cristobalite and tridymite over very short length scales. The spheres of opal in opal-CT are themselves made up of tiny microcrystalline blades of cristobalite and tridymite. Opal-CT has occasionally been further subdivided in the literature. Water content may be as high as 10 wt%. Lussatite is a synonym. Opal-C, also called Lussatine, is interpreted as consisting of localized order of α-cristobalite with a lot of stacking disorder. Typical water content is about 1.5wt%.
Non-crystalline opal
Two broad categories of non-crystalline opals, sometimes just referred to as "opal-A", have been proposed. The first of these is opal-AG consisting of aggregated spheres of silica, with water filling the space in between. Precious opal and potch opal are generally varieties of this, the difference being in the regularity of the sizes of the spheres and their packing. The second "opal-A" is opal-AN or water-containing amorphous silica-glass. Hyalite is another name for this.
Non-crystalline silica in siliceous sediments is reported to gradually transform to opal-CT and then opal-C as a result of diagenesis, due to the increasing overburden pressure in sedimentary rocks, as some of the stacking disorder is removed.[15]
Naming
The word opal is adapted from the Roman term opalus, but the origin of this word is a matter of debate. However, most modern references suggest it is adapted from the Sanskrit word úpala.[16]
References to the gem are made by Pliny the Elder. It is suggested it was adapted it from Ops, the wife of Saturn and goddess of fertility. The portion of Saturnalia devoted to Ops was "Opalia", similar to opalus.
Another common claim that the term is adapted from the Greek word, opillos. This word has two meanings, one is related to "seeing" and forms the basis of the English words like "opaque", the other is "other" as in "alias" and "alter". It is claimed that opalus combined these uses, meaning "to see a change in color". However, historians have noted that the first appearances of opillos do not occur until after the Romans had taken over the Greek states in 180 B.C., and they had previously used the term paederos.[16]
However, the argument for the Sanskrit origin is strong. The term first appears in Roman references around 250 B.C., at a time when the opal was valued above all other gems. The opals were supplied by traders from the Bosporus, who claimed the gems were being supplied from India. Before this the stone was referred to by a variety of names, but these fell from use after 250.
Historical superstitions
In the Middle Ages, opal was considered a stone that could provide great luck because it was believed to possess all the virtues of each gemstone whose color was represented in the color spectrum of the opal.[17] It was also said to confer the power of invisibility if wrapped in a fresh bay leaf and held in the hand.[17][18] Following the publication of Sir Walter Scott's Anne of Geierstein in 1829, however, opal acquired a less auspicious reputation. In Scott's novel, the Baroness of Arnheim wears an opal talisman with supernatural powers. When a drop of holy water falls on the talisman, the opal turns into a colorless stone and the Baroness dies soon thereafter. Due to the popularity of Scott's novel, people began to associate opals with bad luck and death.[17] Within a year of the publishing of Scott's novel in April 1829, the sale of opals in Europe dropped by 50%, and remained low for the next twenty years or so.[19]
Even as recently as the beginning of the 20th century, it was believed that when a Russian saw an opal among other goods offered for sale, he or she should not buy anything more since the opal was believed to embody the evil eye.[17]
Opal is considered the birthstone for people born in October or under the sign of Scorpio and Libra.
Famous opals
- The Olympic Australis, The world's largest and most valuable gem opal[8]
- The Andamooka Opal, presented to Queen Elizabeth II, also known as the Queen's Opal
- "The Burning of Troy", an opal presented to Joséphine de Beauharnais by Napoleon I of France[20]
- The Flame Queen Opal
- The Halley's Comet Opal, the world's largest uncut black opal
- The clock faces above the information stand in Grand Central Terminal Manhattan, New York
- The Roebling Opal, Smithsonian Institution[21]
- The Galaxy Opal – The World's Largest Polished Opal – 1992 The Guinness Book of Records
See also
- Optical phenomena
- Opalite
References
- ^ a b c d e f g h i j k l m Gemological Institute of America, GIA Gem Reference Guide 1995, ISBN 0-87311-019-6
- ^ "Opal". Webmineral. http://webmineral.com/data/Opal.shtml. Retrieved 2011-10-08.
- ^ "Opal". Mindat.org. http://mindat.org/min-3004.html. Retrieved 2011-10-08.
- ^ http://www.itsanhonour.gov.au/symbols/gemstone.cfm
- ^ a b Klein, Cornelis, and Hurlbut, Cornelius S.; 1985, Manual of Mineralogy, 20th ed., ISBN 0-471-80580-7
- ^ Gribble, C. D. (1988). "Tektosilicates (framework silicates)". Rutley's Elements of Mineralogy (27th ed. ed.). London: Unwin Hyman. p. 431. ISBN 0045490112.
- ^ Yowah Nut: Yowah Nut mineral information and data. Mindat.org (2011-02-20). Retrieved on 2011-03-08.
- ^ a b "The Olympic Australis". Altmanncherny.com.au. http://www.altmanncherny.com.au/The%20Olympic%20Australis.htm. Retrieved 2011-10-08.
- ^ Queensland opal[dead link]
- ^ Opal Fossils. Opals Down Under. Retrieved on 2011-03-08.
- ^ "Boi Morto Mine, Pedro II, Piauí, Brazil". Mindat.org. http://www.mindat.org/gallery.php?loc=8782. Retrieved 2011-10-08.
- ^ "NASA probe finds opals in Martian crevices". http://www.jpl.nasa.gov/news/news.cfm?release=2008-198. Retrieved 2008-10-29.
- ^ "Macroporous Structures, Metal Oxides, Highly_Ordered - Office for Technology Commercialization, Express_license, University_of_Minnesota, Technology_Marketing_Site". License.umn.edu. 2010-06-25. http://www.license.umn.edu/Products/Highly-Ordered-Macroporous-Structures__99014.aspx. Retrieved 2011-10-08.
- ^ Graetsch, H., "Structural Characteristics of opaline and microcrystalline silica minerals", "Silica, physical behavior, geochemistry and materials applications". Reviews in Mineralogy, Vol. 29, 1994. Editors PJ Heaney, Connecticut Prewitt, GV Gibbs, Mineralogical Society of America
- ^ "(Cady ''et al.'', 1996)" (PDF). http://minsocam.org/msa/AmMin/TOC/Articles_Free/1996/Cady_p1380-1395_96.pdf. Retrieved 2011-10-08.
- ^ a b Allan Eckert, "The World of Opals", John Wiley and Sons, 1997,pg. 56–57
- ^ a b c d Fernie, William Thomas (1907). Precious Stones for Curative Wear. Bristol, John Wright & Co. pp. 248–249.
- ^ Dunwich, Gerina (1996). Wicca Candle Magick. pp. 84–85.
- ^ Eckert, Allan W. “A Chronological History and Mythology of Opals.” In: The world of opals. New York: John Wiley & Sons, 1997. See pages 53-118.
- ^ Eckert, Allan W. (1997). The world of opals. Chichester: John Wiley & Sons. ISBN 0-471-13397-3.
- ^ "The Dynamic Earth @ National Museum of Natural History". Mnh.si.edu. http://www.mnh.si.edu/earth/text/dynamicearth/6_0_0_GeoGallery/geogallery_specimen.cfm?SpecimenID=4038&categoryID=1&categoryName=Gems&browseType=name. Retrieved 2011-10-08.
External links
*Farlang opal Hist. References Localities, anecdotes by Theophrastus, Isaac Newton, Georg Agricola etc.
- ICA's Opal Page: International Colored Stone Association
Jewellery Forms Anklet · Belt buckle · Belly chain · Bindi · Bracelet · Brooch · Chatelaine · Collar pin · Crown · Cufflink · Earring · Lapel pin · Necklace · Pendant · Ring · Tiara · Tie clip · Toe ring · Watch (pocket)Making PeopleProcessesCasting (centrifugal, lost-wax, vacuum) · Enameling · Engraving · Filigree · Metal clay · Plating · Polishing · Repoussé and chasing · Soldering · Stonesetting · Wire wrappingToolsMaterials Precious metal alloysBritannia silver · Colored gold · Crown gold · Electrum · Platinum sterling · Shakudo · Shibuichi · Sterling silver · TumbagaMineral gemstonesAventurine · Agate · Alexandrite · Amethyst · Aquamarine · Carnelian · Citrine · Diamond · Diopside · Emerald · Garnet · Jade · Jasper · Lapis lazuli · Larimar · Malachite · Marcasite · Moonstone · Obsidian · Onyx · Opal · Peridot · Quartz · Ruby · Sapphire · Sodalite · Sunstone · Tanzanite · Tiger's eye · Topaz · Tourmaline · TurquoiseOrganic gemstonesTerms Crystalline Cryptocrystalline Amorphous Miscellaneous Notable varieties OpalCategories:- Gemstones
- Glass in nature
- Hydrates
- National symbols of Australia
- Quartz varieties
Wikimedia Foundation. 2010.