- Selective glucocorticoid receptor agonist
-
"DIGRA" redirects here. For the Digital Games Research Association, see DiGRA.
A selective glucocorticoid receptor agonist (SEGRA), sometimes called a dissociated glucocorticoid receptor agonist (DIGRA), is a type of experimental drug that is designed to share many of the desirable anti-inflammatory and immunosuppressive properties of classical glucocorticoid drugs but with fewer side effects such as skin atrophy.[2][3] As of November 2010[update], Phase II clinical trials with the SEGRA mapracorat[4] investigating the topical treatment of atopic dermatitis[5] and inflammation following cataract surgery[6] have been completed, but no results are available. Such trials attempt to determine whether the theoretical concept translates into an actual clinical benefit.
Contents
History
Synthetic steroids with SEGRA-like properties were already discovered in the late 1990s.[7] During the 2000s, many potential SEGRAs were synthesized, most of them having non-steroidal structures. Compounds were investigated in cellular models, which established that these molecules bind to the glucocorticoid receptor with an affinity similar to dexamethasone, a potent glucocorticoid, and that they are able to repress the production of inflammatory mediators such as interleukin 6 and prostaglandin E2.[8] Studies in mice showed that a topically administered SEGRA inhibited peroxidase activity and formation of oedema, both indicators of anti-inflammatory activity, comparably to prednisolone. Skin atrophy in rats was significantly less pronounced than under prednisolone in the same study, and metabolic effects like weight gain or increase of blood glucose were practically inexistent.[9]
Phase II clinical trials with one of the candidate compounds, mapracorat (code names BOL-303242-X and ZK 245186[10]), started in summer 2009. One was a double blind dose finding study for an ointment against atopic dermatitis conducted by Intendis, a part of Bayer HealthCare Pharmaceuticals specialized on dermatology.[5] A Phase III trial started in November 2010, evaluating an ophthalmic suspension for the treatment of inflammation following cataract surgery, conducted by Bausch & Lomb.[6]
Mechanism of action
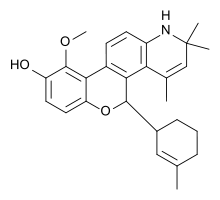 The benzopyranoquinoline A 276575, an example of a SEGRA with a more corticosteroid-like structure[8]
The benzopyranoquinoline A 276575, an example of a SEGRA with a more corticosteroid-like structure[8]
Both non-selective glucocorticoids and selective glucocorticoid receptor agonists work by binding to and activating the glucocorticoid receptor (GR). In contrast to glucocorticoids, which activate the GR to work through two signal transduction pathways,[11] SEGRAs activate the GR in such a way that it only operates through one of the two possible pathways.[12]
In the absence of glucocorticoids, the GR resides in the cytosol in an inactive state complexed with heat shock proteins (HSPs). Binding of glucocorticoids to the GR activates the receptor by causing dissociation of the bound HSPs. The activated GR can then regulate gene expression via one of two pathways:[11]
- The first (direct) pathway is called transactivation whereby the activated GR dimerizes, is translocated into the nucleus and binds to specific sequences of DNA called glucocorticoid response elements. The GR/DNA complex recruits other proteins which transcribe downstream DNA into mRNA and eventually protein. Examples of glucocorticoid responsive genes include those that encode annexin A1, angiotensin-converting enzyme, neutral endopeptidase and other anti-inflammatory proteins.
- The second (indirect) pathway is called transrepression, in which activated monomeric GR binds to other transcription factors such as NF-κB and AP-1 and prevents these from up-regulating the expression of their target genes. These target genes encode proteins such as cyclooxygenase, NO synthase, phospholipase A2, tumor necrosis factor, transforming growth factor beta, ICAM-1, and a number of other pro-inflammatory proteins.[13]
Hence the anti-inflammatory effects of glucocorticoids results from both transactivation and transrepression. In contrast, studies in rats have shown that most of the side-effects of glucocorticoids, such as diabetogenic activity, osteoporosis, as well as skin atrophy, are caused by transactivation.[9][13][14] A selective glucocorticoid that is able to transrepress without transactivation should preserve many of the desirable therapeutic anti-inflammatory effects and minimize undesired side effects.[12]
Strong evidence that transpression alone is sufficient for an anti-inflammatory response was provided by introducing a point mutation in the GR of mice that prevented GR from dimerizing and binding to DNA and thereby blocking transactivation.[15][16] At same time, this mutation did not interfere with transrepression. While GR is essential for survival, these mice are still viable.[15] However, when these mice were treated with the synthetic glucocorticoid dexamethasone, there was no elevation of glucose. At the same time, these dexamethsone treated mice were resistant to inflammatory stimulus.[16] Hence these mice were responsive to the anti-inflammatory effects of dexamethasone but were resistant to at least some of the side-effects.
Just like glucocorticoids, SEGRAs bind to and activate GR. However in contrast to glucocorticoids, SEGRAs selectively activate the GR such that they more strongly transrepress than transactivate. This should result in fewer side effecs.[17]
Potential applications
In chronic inflammatory diseases of the skin like atopic dermatitis, the side-effects of corticoids are problematic because of the necessary long-term treatment. Therefore, SEGRAs are being investigated as an alternative topical treatment. Systemic long-term treatment of inflammations with corticoids is particularly liable to cause metabolic side-effects, which makes the development of oral SEGRAs an interesting goal.[18] Inflammatory diseases of the eye are also a potential application because corticoids can promote glaucoma, cataract, and eye infections.[19] It remains to be seen whether selective receptor agonists cause significantly less side-effects than classical corticoids in clinical application.
The atrophic effects of glucocorticoids are not always a disadvantage. The treatment of hyperproliferative diseases like psoriasis makes use of this property.[20] SEGRAs would likely be less effective in such conditions.
Chemistry
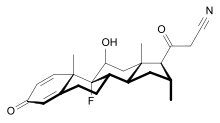 RU 24858, a SEGRA with steroid structure[7]
RU 24858, a SEGRA with steroid structure[7]
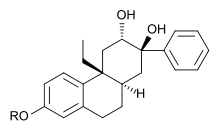 An octahydrophenanthrene-2,7-diol derivative with SEGRA properties[2]
An octahydrophenanthrene-2,7-diol derivative with SEGRA properties[2]
Early SEGRAs were synthetic steroids. An example is RU 24858, one of the first compounds of this type to be published.[7] Many newer SEGRAs have a different framework, although the similarity to steroids can still be seen in molecules like the benzopyranoquinoline A 276575 or in octahydrophenanthrene-2,7-diol derivatives. All of these compounds have been shown to exhibit SEGRA properties in cellular or in animal models.[2]
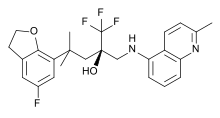
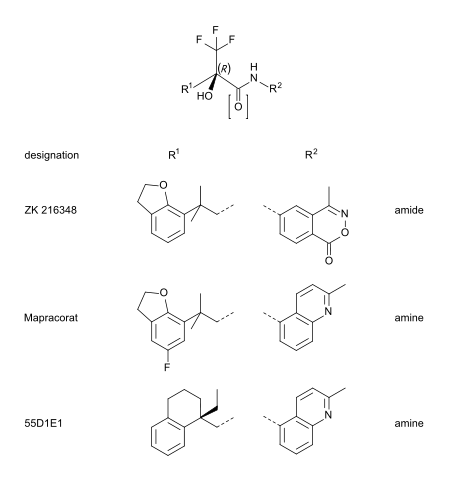
Mapracorat is one of a number of trifluoropropanolamines and -amides which are less obviously steroid-like in structure. Other typical examples of this group are ZK 216348[9] and 55D1E1[3]. The bulky, bicyclic aromatic substituents (R1 and R2) account for the structural similarity to corticoids. The R conformation of the asymmetric carbon atom seems to be essential for GR affinity.[9]
See also
- Selective receptor modulator
- Selective androgen receptor modulator
- Selective estrogen receptor modulator
- Selective progesterone receptor modulator
References
- ^ Mealy, N; Dulsat, C (2009). "36th Annual Meeting of the Arbeitsgemeinschaft Dermatologische Forschung (ADF)". Drugs Fut 34 (4): 341.
- ^ a b c Robinson, RP; Buckbinder, L; Haugeto, AI; McNiff, PA; Millham, ML; Reese, MR; Schaefer, JF; Abramov, YA et al. (2009). "Octahydrophenanthrene-2,7-diol analogues as dissociated glucocorticoid receptor agonists: discovery and lead exploration". Journal of medicinal chemistry 52 (6): 1731–43. doi:10.1021/jm801512v. PMID 19239259.
- ^ a b Biggadike, Keith; Boudjelal, Mohamed; Clackers, Margaret; Coe, Diane M.; Demaine, Derek A.; Hardy, George W.; Humphreys, Davina; Inglis, Graham G. A. et al. (2007). "Nonsteroidal Glucocorticoid Agonists: Tetrahydronaphthalenes with Alternative Steroidal A-Ring Mimetics Possessing Dissociated (Transrepression/Transactivation) Efficacy Selectivity". Journal of Medicinal Chemistry 50 (26): 6519–34. doi:10.1021/jm070778w. PMID 18038970.
- ^ Zhang, Jin-Zhong; Cavet, ME; Vandermeid, KR; Salvador-Silva, M; López, FJ; Ward, KW (2009). "BOL-303242-X, a novel selective glucocorticoid receptor agonist, with full anti-inflammatory properties in human ocular cells". Molecular Vision 15: 2606–16. PMC 2790480. PMID 20011631. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2790480.
- ^ a b ClinicalTrials.gov NCT00944632 Dose Escalation of Different Concentrations of ZK 245186 in Atopic Dermatitis
- ^ a b ClinicalTrials.gov NCT01230125 Mapracorat Ophthalmic Suspension for the Treatment of Ocular Inflammation Following Cataract Surgery
- ^ a b c Vayssière, BM; Dupont, S; Choquart, A; Petit, F; Garcia, T; Marchandeau, C; Gronemeyer, H; Resche-Rigon, M (1997). "Synthetic Glucocorticoids That Dissociate Transactivation and AP-1 Transrepression Exhibit Antiinflammatory Activity in Vivo". Molecular Endocrinology 11 (9): 1245–55. doi:10.1210/me.11.9.1245. PMID 9259316.
- ^ a b Lin, CW; Nakane, M; Stashko, M; Falls, D; Kuk, J; Miller, L; Huang, R; Tyree, C; Miner, Jn; Rosen, J; Kym, Pr; Coghlan, Mj; Carter, G; Lane, Bc (Aug 2002). "trans-Activation and repression properties of the novel nonsteroid glucocorticoid receptor ligand 2,5-dihydro-9-hydroxy-10-methoxy-2,2,4-trimethyl-5-(1-methylcyclohexen-3-y1)-1H-1benzopyrano3,4-fquinoline (A276575) and its four stereoisomers" (Free full text). Molecular pharmacology 62 (2): 297–303. doi:10.1124/mol.62.2.297. ISSN 0026-895X. PMID 12130681. http://molpharm.aspetjournals.org/cgi/pmidlookup?view=long&pmid=12130681.
- ^ a b c d Schäcke, H; Schottelius, A; Döcke, Wd; Strehlke, P; Jaroch, S; Schmees, N; Rehwinkel, H; Hennekes, H; Asadullah, K (Jan 2004). "Dissociation of transactivation from transrepression by a selective glucocorticoid receptor agonist leads to separation of therapeutic effects from side effects" (Free full text). Proceedings of the National Academy of Sciences of the United States of America 101 (1): 227–32. doi:10.1073/pnas.0300372101. PMC 314167. PMID 14694204. http://www.pnas.org/cgi/pmidlookup?view=long&pmid=14694204.
- ^ Cavet, ME; Harrington, KL; Ward, KW; Zhang, JZ (2010). "Mapracorat, a novel selective glucocorticoid receptor agonist, inhibits hyperosmolar-induced cytokine release and MAPK pathways in human corneal epithelial cells". Molecular vision 16: 1791–800. PMC 2932489. PMID 20824100. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2932489.
- ^ a b Rhen T, Cidlowski JA (October 2005). "Antiinflammatory action of glucocorticoids--new mechanisms for old drugs". N. Engl. J. Med. 353 (16): 1711–23. doi:10.1056/NEJMra050541. PMID 16236742.
- ^ a b Newton R, Holden NS (October 2007). "Separating transrepression and transactivation: a distressing divorce for the glucocorticoid receptor?". Mol. Pharmacol. 72 (4): 799–809. doi:10.1124/mol.107.038794. PMID 17622575.
- ^ a b Heinemann, Akos; Schuligoi, Rufina (2008). "Glucocorticoide – potent und umstritten [Glucocorticoids – potent and controversial]" (in German). Österreichische Apothekerzeitung 62 (23). http://www.oeaz.at/zeitung/3aktuell/2008/23/haupt/haupt23_2008_gluco.html.
- ^ Coghlan, M. J.; Jacobson, PB; Lane, B; Nakane, M; Lin, CW; Elmore, SW; Kym, PR; Luly, JR et al. (2003). "A Novel Antiinflammatory Maintains Glucocorticoid Efficacy with Reduced Side Effects". Molecular Endocrinology 17 (5): 860–9. doi:10.1210/me.2002-0355. PMID 12586843.
- ^ a b Reichardt HM, Kaestner KH, Tuckermann J, Kretz O, Wessely O, Bock R, Gass P, Schmid W, Herrlich P, Angel P, Schütz G (May 1998). "DNA binding of the glucocorticoid receptor is not essential for survival". Cell 93 (4): 531–41. doi:10.1016/S0092-8674(00)81183-6. PMID 9604929.
- ^ a b Reichardt HM, Tronche F, Bauer A, Schütz G (2000). "Molecular genetic analysis of glucocorticoid signaling using the Cre/loxP system". Biol. Chem. 381 (9–10): 961–4. doi:10.1515/BC.2000.118. PMID 11076028.
- ^ Schäcke, H; Berger, M; Rehwinkel, H; Asadullah, K (Sep 2007). "Selective glucocorticoid receptor agonists (SEGRAs): novel ligands with an improved therapeutic index". Molecular and cellular endocrinology 275 (1–2): 109–17. doi:10.1016/j.mce.2007.05.014. PMID 17630119.
- ^ Schäcke, H; Hennekes, H; Schottelius, A; Jaroch, S; Lehmann, M; Schmees, N; Rehwinkel, H; Asadullah, K (2002). "SEGRAs: a novel class of anti-inflammatory compounds". Ernst Schering Research Foundation workshop (40): 357–71. PMID 12355726.
- ^ Renfro, L; Snow, JS (1992). "Ocular effects of topical and systemic steroids". Dermatologic clinics 10 (3): 505–12. PMID 1617809.
- ^ Kerscher, MJ; Hart, H; Korting, HC; Stalleicken, D (1995). "In vivo assessment of the atrophogenic potency of mometasone furoate, a newly developed chlorinated potent topical glucocorticoid as compared to other topical glucocorticoids old and new". International journal of clinical pharmacology and therapeutics 33 (4): 187–9. PMID 7620686.
Categories:- Anti-inflammatory agents
- Immunosuppressants
Wikimedia Foundation. 2010.