- Levothyroxine
-
Levothyroxine 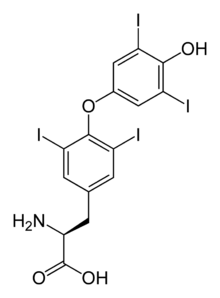

Systematic (IUPAC) name 3,5,3',5'-Tetraiodo-L-thyronine Clinical data Trade names Euthyrox among others, Synthroid, Thyrax AHFS/Drugs.com monograph MedlinePlus a682461 Pregnancy cat. A(US) Legal status ℞ Prescription only Routes Oral, Intravenous Pharmacokinetic data Bioavailability ~100% Metabolism Mainly in liver, kidneys, brain and muscles Half-life ca. 7 days (in hyperthyroidism 3-4 days, in hypothyroidism 9-10 days) Excretion Through feces and urine Identifiers CAS number 51-48-9 
ATC code H03AA01 PubChem CID 5819 DrugBank APRD00235 ChemSpider 5614 
UNII Q51BO43MG4 
ChEBI CHEBI:18332 
ChEMBL CHEMBL1624 
Synonyms O-(4-hydroxy-3,5-diiodophenyl)-3,5-diiodo-L-tyrosine Chemical data Formula C15H11I4NO4 Mol. mass 776.87 SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)Levothyroxine, also L-thyroxine, synthetic T4, or 3,5,3',5'-tetraiodo-L-thyronine, is a synthetic form of thyroxine (thyroid hormone), used as a hormone replacement for patients with thyroid problems. The natural hormone is chemically in the chiral L-form, as is the pharmaceutical agent. Dextrothyroxine (D-thyroxine) briefly saw research as an anticholesterol agent but was pulled due to cardiac side-effects.
Contents
Medical uses
Levothyroxine is typically used to treat hypothyroidism.[1] It may also be used to treat goiter via its ability to lower thyroid-stimulating hormone (TSH), a hormone that is considered goiter-inducing.[2][3]
Adverse effects
Dosing must be carefully controlled to achieve TSH levels within the normal reference range. Long-term suppression of TSH values below normal values will frequently cause cardiac side-effects and contribute to decreases in bone mineral density (high TSH levels are also well known to contribute to osteoporosis).[4]
Patients prescribed too high a dose of levothyroxine may experience effects that mimic hyperthyroidism.[5] Overdose can result in heart palpitations, abdominal pain, nausea, anxiousness, confusion, agitation, insomnia, weight loss, and increased appetite.[6] Allergic reactions to the drug are characterized by symptoms such as difficulty breathing, shortness of breath, or swelling of the face and tongue. Acute overdose may cause fever, hypoglycemia, heart failure, coma and unrecognized adrenal insufficiency.
Acute massive overdose may be life-threatening; treatment should be symptomatic and supportive. Massive overdose may require beta-blockers for increased sympathomimetic activity.[5]
The effects of overdosing appear 6 hours to 11 days after ingestion.[6]
Interactions
There are also foods and other substances that can interfere with absorption of thyroxine replacement. People ought to avoid taking calcium and iron supplements within 4 hours,[7] as well as soy products within 3 hours of the medication, as these can reduce absorption of the drug. Grapefruit juice may delay the absorption of levothyroxine, but it is not believed to have a significant effect on bioavailability.[8] Other substances that reduce absorption are aluminium and magnesium containing antacids, simethicone or sucralfate, cholestyramine, colestipol, Kayexalate. A study of eight women suggested that coffee may interfere with the intestinal absorption of levothyroxine, though at a level less than eating bran.[9] Different substances cause other adverse effects that may be severe. Ketamine may cause hypertension and tachycardia and tricyclic and tetracyclic antidepressants increase its toxicity. On the other hand lithium can cause hyperthyroidism (but most often hypothyroidism) by affecting iodine metabolism of the thyroid itself and thus inhibits synthetic levothyroxine as well.
Dosage
Dosages vary according to the age groups and the individual condition of the patient, body weight and compliance to the medication and diet. Monitoring of the patient's condition and adjustment of the dosage is periodical and necessary. Levothyroxine is taken on an empty stomach approximately half an hour to an hour before meals.[5]
Brand names
Common brand names include Thyrax, Euthyrox, Levaxin, L-thyroxine, Eltroxin and Thyrax Duotab in Europe; Thyrox in South Asia; Eutirox, Tirosint, Levoxyl and Synthroid in North America. There are also numerous generic versions.
References
- ^ Vaidya B, Pearce SH (2008). "Management of hypothyroidism in adults". BMJ (Clinical research ed.) 337: a801. doi:10.1136/bmj.a801. PMID 18662921. http://bmj.com/cgi/pmidlookup?view=long&pmid=18662921.
- ^ Svensson J, Ericsson UB, Nilsson P, et al. (May 2006). "Levothyroxine treatment reduces thyroid size in children and adolescents with chronic autoimmune thyroiditis". The Journal of clinical endocrinology and metabolism 91 (5): 1729–34. doi:10.1210/jc.2005-2400. PMID 16507633. http://jcem.endojournals.org/cgi/pmidlookup?view=long&pmid=16507633.
- ^ Dietlein, M; Wegscheider, K; Vaupel, R; Schmidt, M; Schicha, H (2007). "Management of multinodular goiter in Germany (Papillon 2005): do the approaches of thyroid specialists and primary care practitioners differ?". Nuklearmedizin. Nuclear medicine 46 (3): 65–75. PMID 17549317.
- ^ Frilling, A; Liu, C; Weber, F (2004). "Benign multinodular goiter". Scandinavian journal of surgery : SJS : official organ for the Finnish Surgical Society and the Scandinavian Surgical Society 93 (4): 278–81. PMID 15658668.
- ^ a b c "Synthroid (Levothyroxine Sodium) Drug Information: Uses, Side Effects, Drug Interactions and Warnings". RxList. http://www.rxlist.com/synthroid-drug.htm. Retrieved 2010-07-18.
- ^ a b Irizarry, Lisandro (2010-04-23). "Toxicity, Thyroid Hormone". WebMd. http://www.emedicine.com/emerg/TOPIC800.HTM. Retrieved 2010-10-31.
- ^ Ruth H. Michel, Patricia J. Neafsey, Laura Cox Dzurec: Self Medication Practices among Patients taking Levothyroxine. The Internet Journal of Advanced Nursing Practice. 2004. Volume 6 Number 2
- ^ Lilja JJ, Laitinen K, Neuvonen PJ (September 2005). "Effects of grapefruit juice on the absorption of levothyroxine". Br J Clin Pharmacol 60 (3): 337–41. doi:10.1111/j.1365-2125.2005.02433.x. PMC 1884777. PMID 16120075. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1884777.
- ^ Benvenga, Salvatore; Bartolone, L.; Pappalardo, M.A.; Russo, A.; Lapa, D.; Giorgianni, G.; Saraceno, G.; Trimarchi, F. (March 2008). "Altered Intestinal Absorption of L-Thyroxine Caused by Coffee". Thyroid (New York: Mary Ann Liebert, Inc.) 18 (3): 293–301. doi:10.1089/thy.2007.0222. PMID 18341376. http://www.ncbi.nlm.nih.gov/pubmed/18341376?dopt=Citation. Retrieved 2009-05-16.
External links
- Detailed Euthyrox (Levothroid/Levothyroxine) Consumer Information: Uses, Precautions, Side Effects
- U.S. National Library of Medicine: Drug Information Portal - Levothyroxine
Thyroid therapy (H03) Thyroid hormones Antithyroid preparations Thiouracils: Propylthiouracil# • Methylthiouracil • Benzylthiouracil
Sulfur-containing imidazole derivatives: Carbimazole • MethimazoleOtherCategories:- Hormonal agents
- Iodinated tyrosine derivatives
Wikimedia Foundation. 2010.

