- Carbimazole
-
Carbimazole 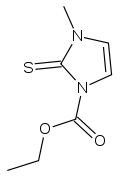
Systematic (IUPAC) name ethyl 3-methyl-2-sulfanylidene-imidazole-1-carboxylate Clinical data Trade names Neo-mercazole AHFS/Drugs.com International Drug Names Pregnancy cat. ? Legal status ℞ Prescription only Routes oral Pharmacokinetic data Protein binding 85% Identifiers CAS number 22232-54-8 ATC code H03BB01 PubChem CID 31072 DrugBank APRD00503 ChemSpider 28829 
UNII 8KQ660G60G 
KEGG D07616 
ChEBI CHEBI:617099 
ChEMBL CHEMBL508102 
Chemical data Formula C7H10N2O2S Mol. mass 186.233 g/mol SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)Carbimazole is used to treat hyperthyroidism. Carbimazole is a pro-drug as after absorption it is converted to the active form, methimazole. Methimazole prevents the thyroid peroxidase enzyme from coupling and iodinating the tyrosine residues on thyroglobulin, hence reducing the production of the thyroid hormones T3 and T4 (thyroxine).
Contents
Clinical use
Therapy for hyperthyroidism generally starts at a high daily dose of 15 - 40mg continued until the patient has normal thyroid function, and then reduced to a maintenance dose of 5 - 15mg. Treatment is usually given for 12 - 18 months followed by a trial withdraw.
The onset of anti-thyroid effect is rapid but the onset of clinical effects on thyroid hormone levels in the blood is much slower. This is because the large store of pre-formed T3 and T4 in the thyroid gland and bound to Thyroid Binding Globulin (99% bound) has to be depleted before any beneficial clinical effect occurs.
Precautions
Some people are allergic to azole(s).
Some azole drugs have adverse side-effects.
Some azole drugs may disrupt estrogen production in pregnancy, affecting pregnancy outcome. [1]
Side Effects
Whilst rashes and pruritus are common, these can often be treated with antihistamines without stopping the carbimazole. For those patients where sensitivity reactions can not be controlled, propylthiouracil may be used as an alternative.
Its most serious rare side effect is bone marrow suppression causing neutropenia and agranulocytosis. This may occur at any stage during treatment and without warning. Patients are advised to immediately report symptoms of infection, especially sore throats, so that a full blood count test may be arranged. If this confirms a low neutrophil count then the drug must be discontinued immediately, allowing for usually a prompt recovery. However failure to report suggestive symptoms or delays in considering the possibility of immunosuppression and its testing, can lead to fatalities. Should be used with caution in pregnancy as crosses the placenta barrier.
Brand names
- Neomercazole
- Vidalta
- Camazol
See also
References
- British National Formulary 45 March 2003
Thyroid therapy (H03) Thyroid hormones Antithyroid preparations Thiouracils: Propylthiouracil# • Methylthiouracil • Benzylthiouracil
Sulfur-containing imidazole derivatives: Carbimazole • MethimazoleOther
This hormonal preparation article is a stub. You can help Wikipedia by expanding it.
