- Cytotoxic T cell
-
A cytotoxic T cell (also known as TC, Cytotoxic T Lymphocyte, CTL, T-Killer cell, cytolytic T cell, CD8+ T-cells or killer T cell) belongs to a sub-group of T lymphocytes (a type of white blood cell) that are capable of inducing the death of infected somatic or tumor cells; they kill cells that are infected with viruses (or other pathogens), or are otherwise damaged or dysfunctional. Most cytotoxic T cells express T-cell receptors (TCRs) that can recognize a specific antigenic peptide bound to Class I MHC molecules, present on all nucleated cells, and a glycoprotein called CD8, which is attracted to non-variable portions of the Class I MHC molecule. The affinity between CD8 and the MHC molecule keeps the TC cell and the target cell bound closely together during antigen-specific activation. CD8+ T cells are recognized as TC cells once they become activated and are generally classified as having a pre-defined cytotoxic role within the immune system. However, CD8+ T cells also have the ability to make some cytokines.
Contents
Development
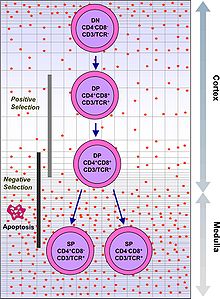
Hematopoietic stem cells in the bone marrow migrate into the thymus, where they undergo VDJ recombination of their beta-chain TCR DNA to form a developmental form of the TCR protein, known as pre-TCR. If that rearrangement is successful, the cells then rearrange their alpha-chain TCR DNA to create a functional alpha-beta TCR complex. This highly-variable genetic rearrangement product in the TCR genes helps create millions of different T cells with different TCRs, helping the body's immune system respond to virtually any protein of an invader. The vast majority of T cells express alpha-beta TCRs (αβ T cells), but some T cells in epithelial tissues (like the gut) express gamma-delta TCRs (γδ T cells), which recognize non-protein antigens.
T cells with functionally stable TCRs express both the CD4 and CD8 co-receptors and are therefore termed "double-positive" (DP) T cells (CD4+CD8+). The double-positive T cells are exposed to a wide variety of self-antigens in the thymus and undergo two selection criteria:
- positive selection, in which those double-positive T cells that bind too weakly to MHC-presented self antigens undergo apoptosis because of their inability to recognize MHC-protein complexes.
- negative selection, in which those double-positive T cells that bind too strongly to MHC-presented self antigens undergo apoptosis because their propensity (an often intense natural inclination or preference) to become autoreactive could lead to autoimmunity.
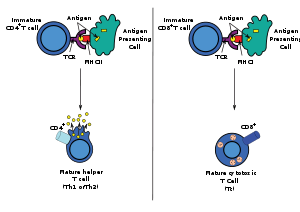
Only those T cells that bind to the MHC-self-antigen complexes weakly are positively selected. Those cells that survive positive and negative selection differentiate into single-positive T cells (either CD4+ or CD8+), depending on whether their TCR recognizes an MHC class I-presented antigen (CD8) or an MHC class II-presented antigen (CD4). It is the CD8+ T-cells that will mature and go on to become cytotoxic T cells following their activation with a class I-restricted antigen.
Activation
With an exception of some cell types, such as non-nucleated cells (including erythrocytes), Class I MHC is expressed by all host cells. When these cells are infected with a virus (or another intracellular pathogen), the cells degrade foreign proteins via antigen processing. These result in peptide fragments, some of which are presented by MHC Class I to the T cell antigen receptor (TCR) on CD8+ T cells.
The activation of cytotoxic T cells is dependent on several simultaneous interactions between molecules expressed on the surface of the T cell and molecules on the surface of the antigen-presenting cell (APC). For instance, consider the two signal model for TC cell activation.
Signal T cell APC Description first signal TCR peptide-bound MHC class I molecule There is a second interaction between the CD8 coreceptor and the class I MHC molecule to stabilize this signal. second signal CD28 molecule on the T cell either CD80 or CD86 (also called B7-1 and B7-2) CD80 and CD86 are known as costimulators for T cell activation. This second signal can be assisted (or replaced) by stimulating the TC cell with cytokines released from helper T cells. While in most cases activation is dependent on TCR recognition of antigen, alternative pathways for activation have been described. For example, cytotoxic T cells have been shown to become activated when targeted by other CD8 T cells leading to tolerization of the latter.[1]
Once activated, the TC cell undergoes clonal expansion with the help of a cytokine called Interleukin-2 (IL-2) that is a growth and differentiation factor for T cells. This increases the number of cells specific for the target antigen that can then travel throughout the body in search of antigen-positive somatic cells.
Effector functions
When exposed to infected/dysfunctional somatic cells, TC cells release the cytotoxins perforin, granzymes, and granulysin. Perforin forms pores (aqueous channels) in the target cell's plasma membrane allowing granzymes, types of serine proteases that cleave at aspartate residues in the substrate, to enter the target cell, which then activate a series of cysteine proteases, the caspase cascade, that eventually lead to apoptosis (programed cell death). A second way to induce apoptosis is via cell-surface interactions between the TC and the infected cell. When a TC is activated it starts to express the surface protein FAS ligand (FasL)(Apo1L)(CD95L), which can bind to Fas (Apo1)(CD95) molecules expressed on the target cell. However, this Fas-Fas ligand interaction is thought to be more important to the disposal of unwanted T lymphocytes during their development or to the lytic activity of certain TH cells than it is to the cytolytic activity of TC effector cells. Engagement of Fas with FasL allows for recruitment of the death-induced signaling complex (DISC). The Fas-associated death domain (FADD) translocates with the DISC, allowing recruitment of procaspases 8 and 10. These caspases then activate the effector caspases 3, 6, and 7, leading to cleavage of death substrates such as lamin A, lamin B1, lamin B2, PARP (poly-ADP ribose polymerase), and DNAPK (DNA-activated protein kinase). The final result is apoptosis of the cell that expressed Fas.
Role in disease pathogenesis
See also: Hepatitis B virusDuring hepatitis B virus (HBV) infection cytotoxic T cells play an important pathogenetic role. They contribute to nearly all of the liver injury associated with HBV infection and, by killing infected cells and by producing antiviral cytokines capable of purging HBV from viable hepatocytes, cytotoxic T cells also eliminate the virus.[2] Recently platelets have been shown to facilitate the accumulation of virus-specific cytotoxic T cells into the infected liver.[3] Recently CTLs have been implicated in the progression of arthritis: depletion of knee joint cartilage macromolecules such as glycosaminoglycans by CTLs and macrophages has been observed in a rat model of the disease.[4]
See also
References
- ^ Milstein, O., Hagin, D., Lask, A., Reich-Zeliger, S., Shezan E., Ophir E., Eidelshtein Y., Afik R., Antebi YE., Dustin ML. and Reisner Y. (2011) CTLs respond with activation and granule secretion when serving target for T cell recognition. Blood 117,1042-1052
- ^ Iannacone, Matteo; Sitia, Giovanni; Guidotti, Luca G (2006). "Pathogenetic and antiviral immune responses against hepatitis B virus". Future Virology 1 (2): 189–96. doi:10.2217/17460794.1.2.189.
- ^ Iannacone, Matteo; Sitia, Giovanni; Isogawa, Masanori; Marchese, Patrizia; Castro, Maria G; Lowenstein, Pedro R; Chisari, Francis V; Ruggeri, Zaverio M et al. (2005). "Platelets mediate cytotoxic T lymphocyte–induced liver damage". Nature Medicine 11 (11): 1167–9. doi:10.1038/nm1317. PMC 2908083. PMID 16258538. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2908083.
- ^ Subramanian S and Ramalingam K (2005). "Electron microscopic evidence on the participation Cytotoxic T Lymphocytes and Macrophages in Mtb adjuvant induced connective tissue inflammation and arthritogenesis in Rattus norvegicus". Asian Journal of Microbiology, Biotechnology and Environmental Sciences 7 (2): 227–233. ISSN 0972-3005.
External links
- T-cell Group - Cardiff University
- Malaria (Flash Animation)
Blood: Lymphocytes Lymphoid/
HSC:CFU-LLymphopoiesis Immunology: Lymphocytic adaptive immune system and complement Lymphoid AntigensAntibodiesImmunity vs.
toleranceaction: Immunity · Autoimmunity · Alloimmunity · Allergy · Hypersensitivity · Inflammation · Cross-reactivity
inaction: Tolerance (Central, Peripheral, Clonal anergy, Clonal deletion, Tolerance in pregnancy) · ImmunodeficiencyLymphocytes Substances Complement Categories:- T cells
- Human cells
Wikimedia Foundation. 2010.