- Methylglyoxal
-
Methylglyoxal 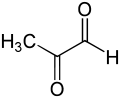
 2-oxopropanal
2-oxopropanalIdentifiers CAS number 78-98-8 
PubChem 880 ChemSpider 857 
UNII 722KLD7415 
DrugBank DB03587 KEGG C00546 
MeSH Methylglyoxal ChEBI CHEBI:17158 
ChEMBL CHEMBL170721 
Jmol-3D images Image 1 - CC(=O)C=O
Properties Molecular formula C3H4O2 Molar mass 72.0627 Related compounds Related ketones, aldehydes glyoxal
propionaldehyde
propanedial
acetone
diacetyl
acetylacetoneRelated compounds glyoxylic acid
pyruvic acid
acetoacetic acid (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Methylglyoxal, also called pyruvaldehyde or 2-oxopropanal (CH3-CO-CH=O or C3H4O2) is the aldehyde form of pyruvic acid. It has two carbonyl groups, so it is a dicarbonyl compound. Methylglyoxal is both an aldehyde and a ketone.
In organisms, methylglyoxal is formed as a side-product of several metabolic pathways.[1] It may form from 3-amino acetone, which is an intermediate of threonine catabolism, as well as through lipid peroxidation. However, the most important source is glycolysis. Here, methylglyoxal arises from nonenzymatic phosphate elimination from glyceraldehyde phosphate and dihydroxyacetone phosphate, two intermediates of glycolysis. Since methylglyoxal is highly cytotoxic, the body developed several detoxification mechanisms. One of these is the glyoxalase system. Methylglyoxal reacts with glutathione to form a hemithioacetal. This is converted into S-D-lactoyl-glutathione by glyoxalase I,[2] and then further metabolised into D-lactate by glyoxalase II.[3]
Why methylglyoxal is produced remains unknown, but several articles indicate it is involved in the formation of advanced glycation endproducts (AGEs). In fact, methylglyoxal is proven to be the most important glycation agent (forming AGEs).[4] In this process, methylglyoxal reacts with free amino groups of lysine and arginine and with thiol groups of cysteine, forming AGEs. Recent research has identified heat shock protein 27 (Hsp27) as a specific target of posttranslational modification by methylglyoxal in human metastatic melanoma cells.[5] Other glycation agents include the reducing sugars:
- glucose, the sugar that stores energy
- galactose, a part of milk sugar (lactose)
- allose, an all-cis hexose carried into the cell by special proteins
- ribose, a component of RNA.
Due to increased blood glucose levels, methylglyoxal has higher concentrations in diabetics, and has been linked to arterial atherogenesis. Damage by methylglyoxal to low-density lipoprotein though glycation causes a fourfold increase of atherogenesis in diabetics.[6]
References
- ^ Inoue Y, Kimura A (1995). "Methylglyoxal and regulation of its metabolism in microorganisms". Adv. Microb. Physiol. 37: 177–227. doi:10.1016/S0065-2911(08)60146-0. PMID 8540421.
- ^ Thornalley PJ (2003). "Glyoxalase I--structure, function and a critical role in the enzymatic defence against glycation". Biochem. Soc. Trans. 31 (Pt 6): 1343–8. doi:10.1042/BST0311343. PMID 14641060. http://www.biochemsoctrans.org/bst/031/1343/bst0311343.htm.
- ^ Vander Jagt DL (1993). "Glyoxalase II: molecular characteristics, kinetics and mechanism". Biochem. Soc. Trans. 21 (2): 522–7. PMID 8359524.
- ^ Shinohara M; Thornalley, PJ; Giardino, I; Beisswenger, P; Thorpe, SR; Onorato, J; Brownlee, M (1998). "Overexpression of glyoxalase-I in bovine endothelial cells inhibits intracellular advanced glycation endproduct formation and prevents hyperglycemia-induced increases in macromolecular endocytosis.". J Clin Invest. 101 (5): 1142–7. doi:10.1172/JCI119885. PMC 508666. PMID 9486985. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=508666.
- ^ Bair WB 3rd, Cabello CM, Uchida K, Bause AS, Wondrak GT (April 2010). "GLO1 overexpression in human malignant melanoma". Melanoma Res 20 (2): 85–96. doi:10.1097/CMR.0b013e3283364903. PMC 2891514. PMID 20093988. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2891514.
- ^ Rabbani N; Godfrey, L; Xue, M; Shaheen, F; Geoffrion, M; Milne, R; Thornalley, PJ (2011 May 26). "Glycation of LDL by methylglyoxal increases arterial atherogenicity. A possible contributor to increased risk of cardiovascular disease in diabetes.". Diabetes. doi:10.2337/db11-0085. PMID 21617182.
See also
Categories:- Aldehydes
- Ketones
- Metabolism
Wikimedia Foundation. 2010.
