- Delta endotoxin
-
delta endotoxin, N-terminal domain 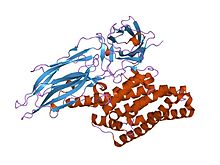
crystal structure of the insecticidal bacterial del endotoxin cry3bb1 bacillus thuringiensis Identifiers Symbol Endotoxin_N Pfam PF03945 InterPro IPR005639 SCOP 1dlc TCDB 1.C.2 Available protein structures: Pfam structures PDB RCSB PDB; PDBe PDBsum structure summary delta endotoxin 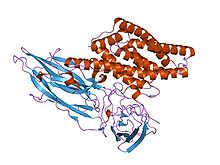
Structure of insecticidal delta-endotoxin from Bacillus thuringiensis.[1] Identifiers Symbol Endotoxin_M Pfam PF00555 InterPro IPR015790 SCOP 1dlc TCDB 1.C.2 OPM family 95 OPM protein 1w99 Available protein structures: Pfam structures PDB RCSB PDB; PDBe PDBsum structure summary Bacillus thuringiensis delta-Endotoxin, middle domain 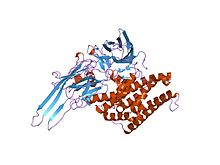
insecticidal crystal protein cry2aa Identifiers Symbol Endotoxin_mid Pfam PF09131 InterPro IPR015214 SCOP 1i5p Available protein structures: Pfam structures PDB RCSB PDB; PDBe PDBsum structure summary delta endotoxin 
insecticidal crystal protein cry2aa Identifiers Symbol Endotoxin_C Pfam PF03944 Pfam clan CL0202 InterPro IPR005638 SCOP 1dlc TCDB 1.C.2 Available protein structures: Pfam structures PDB RCSB PDB; PDBe PDBsum structure summary Delta endotoxins (δ-endotoxins, also called Cry and Cyt toxins) are pore-forming toxins produced by Bacillus thuringiensis species of bacteria. They are useful for their insecticidal action.
During spore formation the bacteria produce crystals of this protein. When an insect ingests these proteins, they are activated by proteolytic cleavage. The N-terminus is cleaved in all of the proteins and a C-terminal extension is cleaved in some members. Once activated, the endotoxin binds to the gut epithelium and causes cell lysis by the formation of cation-selective channels, which leads to death. The activated region of the delta toxin is composed of three distinct structural domains: an N-terminal helical bundle domain (IPR005639) involved in membrane insertion and pore formation; a beta-sheet central domain involved in receptor binding; and a C-terminal beta-sandwich domain (IPR005638) that interacts with the N-terminal domain to form a channel.[2][3][4][5]
References
- ^ Li JD, Carroll J, Ellar DJ (October 1991). "Crystal structure of insecticidal delta-endotoxin from Bacillus thuringiensis at 2.5 A resolution". Nature 353 (6347): 815–21. doi:10.1038/353815a0. PMID 1658659.
- ^ Cygler M, Borisova S, Grochulski P, Masson L, Pusztai-carey M, Schwartz JL, Brousseau R (1995). "Bacillus thuringiensis CryIA(a) insecticidal toxin: crystal structure and channel formation". J. Mol. Biol. 254 (3): 447–464. doi:10.1006/jmbi.1995.0630. PMID 7490762.
- ^ Ghosh D, Pangborn W, Galitsky N, Cody V, Wojtczak A, Luft JR, English L (2001). "Structure of the insecticidal bacterial delta-endotoxinCry3Bb1 of Bacillus thuringiensis". Acta Crystallogr. D 57 (8): 1101–1109. doi:10.1107/S0907444901008186. PMID 11468393.
- ^ Grochulski P, Masson L, Borisova S, Pusztai-Carey M, Schwartz JL, Brousseau R, Cygler M (December 1995). "Bacillus thuringiensis CryIA(a) insecticidal toxin: crystal structure and channel formation". J. Mol. Biol. 254 (3): 447–64. doi:10.1006/jmbi.1995.0630. PMID 7490762.
- ^ Galitsky N, Cody V, Wojtczak A, Ghosh D, Luft JR, Pangborn W, English L (August 2001). "Structure of the insecticidal bacterial delta-endotoxin Cry3Bb1 of Bacillus thuringiensis". Acta Crystallogr. D Biol. Crystallogr. 57 (Pt 8): 1101–9. doi:10.1107/S0907444901008186. PMID 11468393.
Further reading
- Bravo, A.; Gill, S. S.; Soberón, M. (2007). "Mode of action of Bacillus thuringiensis Cry and Cyt toxins and their potential for insect control". Toxicon 49 (4): 423–435. doi:10.1016/j.toxicon.2006.11.022. PMC 1857359. PMID 17198720. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1857359.
- Pigott, C. R.; Ellar, D. J. (2007). "Role of Receptors in Bacillus thuringiensis Crystal Toxin Activity". Microbiology and Molecular Biology Reviews 71 (2): 255–281. doi:10.1128/MMBR.00034-06. PMC 1899880. PMID 17554045. http://mmbr.asm.org/cgi/reprint/71/2/255.pdf.
This article includes text from the public domain Pfam and InterPro IPR015790

This protein-related article is a stub. You can help Wikipedia by expanding it.
