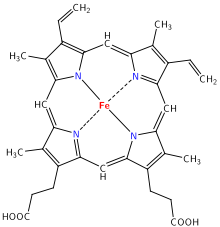
- Dioxygen in biological reactions
-
Further information: Geological history of oxygen
Dioxygen (O2) plays an important role in the energy metabolism of living organisms. Free oxygen is produced in the biosphere through photolysis (light-driven oxidation and splitting) of water during photosynthesis in cyanobacteria, green algae, and plants. During oxidative phosphorylation in cellular respiration, oxygen is reduced to water, thus closing the biological water-oxygen redox cycle.
Contents
Photosynthesis
In nature, free oxygen is produced by the light-driven splitting of water during oxygenic photosynthesis. Green algae and cyanobacteria in marine environments provide about 70% of the free oxygen produced on earth.[1][Need quotation to verify] The remainder is produced by terrestrial plants, although for example, almost all oxygen produced in tropical forests is consumed by organisms living there.[2]
A simplified overall formula for photosynthesis is:[3]
-
- 6CO2 + 6H2O + photons → C6H12O6 + 6O2
(or simply carbon dioxide + water + sunlight → glucose + oxygen)
Photolytic oxygen evolution during photosynthesis occurs via the light-dependent oxidation of water to molecular oxygen and can be written as the following simplified chemical reaction: 2H2O → 4e− + 4H+ + O2
The reaction occurs in the thylakoid membranes of cyanobacteria, and algal and plant chloroplasts and requires the energy of four photons. The electrons from the oxidized water molecules replace electrons in the P680 component of photosystem II, which have been removed into an electron transport chain via light-dependent excitation and resonance energy transfer onto plastoquinone.[4] Photosytem II therefore has also been referred to as water-plastoquinone oxido-reductase.[5] The protons from the oxidized water molecules are released into the thylakoid lumen, thus contributing to the generation of a proton gradient across the thylakoid membrane. This proton gradient is the driving force for ATP synthesis via photophosphorylation and coupling the absorption of light energy and photolysis of water to the creation of chemical energy during photosynthesis.[4] The O2 remaining after oxidation of the water molecule is released into the atmosphere.
Water oxidation is catalyzed by a manganese-containing enzyme complex known as the oxygen evolving complex (OEC) or water-splitting complex found associated with the lumenal side of thylakoid membranes. Manganese is an important cofactor, and calcium and chloride are also required for the reaction to occur.[4]
Oxygen uptake and transport
In vertebrates, oxygen uptake is carried out by the following processes:
Oxygen diffuses through membranes and into red blood cells after inhalation into the lungs. They are bound to dioxygen complexes, which are coordination compounds that contain O2 as a ligand,[6] providing a more efficient oxygen-loading capacity. In blood, the heme group of hemoglobin binds oxygen when it is present, changing hemoglobin's color from bluish red to bright red.[7][8] Vertebrate animals use hemoglobin in their blood to transport oxygen from their lungs to their tissues, but other animals use hemocyanin (molluscs and some arthropods) or hemerythrin (spiders and lobsters).[9][10][11] A liter of blood can dissolve 200 cc of oxygen gas, which is much more than water can dissolve (see Physical Properties).[9]
After being carried in blood to a body tissue in need of oxygen, O2 is handed-off from the heme group to monooxygenase, an enzyme that also has an active site with an atom of iron.[9] Monooxygenase uses oxygen to catalyze many oxidation reactions in the body. Carbon dioxide, a waste product, is released from the cell and into the blood, where it combines with bicarbonate and hemoglobin for transport to the lungs. Blood circulates back to the lungs and the process repeats.[12]
Aerobic respiration
See also: Aerobic respirationMolecular oxygen, O2, is essential for cellular respiration in all aerobic organisms. Oxygen is used as an electron acceptor in mitochondria to generate chemical energy in the form of adenosine triphosphate (ATP) during oxidative phosphorylation. The reaction for the aerobic respiration is essentially the reverse of photosynthesis, except that now there is a large release of chemical energy that is stored in ATP molecules (up to 38 ATP molecules are formed from one molecule of glucose). The simplified version of this reaction is:
-
- C6H12O6 + 6O2 → 6CO2 + 6H2O + 2880 kJ/mol
Reactive oxygen species
Main article: Reactive oxygen speciesReactive oxygen species are dangerous by-products that sometimes result from the use of oxygen in organisms. Important examples include; oxygen free radicals such as the highly-dangerous superoxide O2-, and the less harmful hydrogen peroxide (H2O2).[9] The body uses superoxide dismutase to reduce superoxide radicals to hydrogen peroxide. Glutathione peroxidase and similar enzymes then convert the H2O2 to water and dioxygen.[9]
Parts of the immune system of higher organisms, however, create peroxide, superoxide, and singlet oxygen to destroy invading microbes. Recently, singlet oxygen has been found to be a source of biologically-produced ozone: This reaction proceeds through an unusual compound dihydrogen trioxide, also known as trioxidane, (HOOOH), which is an antibody-catalyzed product of singlet oxygen and water. This compound, in turn, disproportionates to ozone and peroxide, providing two powerful antibacterials. The body's range of defense against all of these active oxidizing agents is hardly surprising, then, given their "deliberate" employment as antimicrobial agents in the immune response.[13] Reactive oxygen species also play an important role in the hypersensitive response of plants against pathogen attack.[4]
Notes
- ^ Fenical, William (September 1983). "Marine Plants: A Unique and Unexplored Resource". Plants: the potentials for extracting protein, medicines, and other useful chemicals (workshop proceedings). DIANE Publishing. pp. 147. ISBN 1428923977. http://books.google.com/?id=g6RfkqCUQyQC&pg=PA147&dq=oxygen+percent+algae+plants.
- ^ Broeker, W.S. (2006). "Breathing easy, Et tu, O2". Columbia University. http://www.columbia.edu/cu/21stC/issue-2.1/broecker.htm. Retrieved 2007-10-21.
- ^ Brown, LeMay, Burslen, Chemistry The Central Science, ISBN 0-13-048450-4, p. 958
- ^ a b c d Raven, Peter H.; Ray F. Evert, Susan E. Eichhorn (2005). Biology of Plants, 7th Edition. New York: W.H. Freeman and Company Publishers. pp. 115–127. ISBN 0-7167-1007-2.
- ^ Raval M, Biswal B, Biswal U (2005). "The mystery of oxygen evolution: analysis of structure and function of photosystem II, the water-plastoquinone oxido-reductase". Photosynthesis Research 85 (3): 267–93. doi:10.1007/s11120-005-8163-4. PMID 16170631.
- ^ Holleman, A. F.; Wiberg, E. "Inorganic Chemistry" Academic Press: San Diego, 2001. ISBN 0-12-352651-5.
- ^ CO2 is released from another part of the hemoglobin molecule, as its acid, which causes CO2 to be released from bicarbonate, its major reservoir in blood plasma (see Bohr effect)
- ^ Stwertka 1998, p. 48.
- ^ a b c d e Emsley 2001, p. 298.
- ^ Cook & Lauer 1968, p. 500.
- ^ Figures given are for values up to 50 miles above the surface
- ^ Emsley 2001, p. 303.
- ^ Hoffmann, Roald (2004). "The Story of O". American Scientist 92 (1): 23. doi:10.1511/2004.1.23. Archived from the original on 2007-02-22. http://web.archive.org/web/20070222052300/http://www.americanscientist.org/template/AssetDetail/assetid/29647?&print=yes. Retrieved 2007-03-03.
References
- Emsley, John (2001). "Oxygen". Nature's Building Blocks: An A-Z Guide to the Elements. Oxford, England, UK: Oxford University Press. pp. 297–304. ISBN 0198503407.
- Cook, Gerhard A.; Lauer, Carol M. (1968). "Oxygen". In Clifford A. Hampel. The Encyclopedia of the Chemical Elements. New York: Reinhold Book Corporation. pp. 499–512. LCCN 68-29938.
- Stwertka, Albert (1998). Guide to the Elements (Revised ed.). Oxford University Press. ISBN 0-19-508083-1.
See also
Categories: -
Wikimedia Foundation. 2010.