- Oxo ligand
-
In coordination chemistry, an oxo ligand is an oxygen atom bound only to one or more metal centers. These ligands can exist as terminal or (most commonly) as bridging atom (Fig. 1). Oxo ligands stabilize high oxidation states of a metal.[1]
 Fig. 1 a) Doubly bridging and b) terminal oxo ligands.
Fig. 1 a) Doubly bridging and b) terminal oxo ligands.
Oxo ligands are pervasive, comprising the great majority of the Earth's crust. This article concerns a subset of oxides, molecular derivatives. They are also found in several metalloenzymes, e.g. in the molybdenum cofactor and in many iron-containing enzymes. One of the earliest synthetic compounds to incorporate an oxo ligand is sodium ferrate (Na2FeO4) circa 1702.[2]
Contents
Reactivity
Olation and acid-base reactions
A common reactions effected by metal-oxo compounds is olation, the condensation process that converts low molecular weight oxides to polymeric materials, including minerals. Olation often begins with the deprotonation of a metal-hydroxo complex.
Oxo-atom transfer
Oxygen-atom transfer is another common reaction of particular interest in organic chemistry,[3] but metal-oxides are capable of a variety of reactions including catalytic process. Examples here demonstrate the range of reactions of molecular metal oxo species. The formation of O2 by the oxygen evolving center can be viewed as an O-atom transfer from a manganese oxo group with water.
Molecular oxides
Some of the longest known and most widely used oxo compounds are oxidizing agents such as potassium permanganate (KMnO4) and osmium tetroxide (OsO4). Compounds such as these have been known since the 1700s and are widely used in organic synthesis, e.g. for converting alkenes to vicinal diols and alcohols to ketones or carboxylic acids.[1] More selective or gentler oxidizing reagents include pyridinium chlorochromate (PCC) and pyridinium dichromate (PDC), which have become widely used since the 1970s.[1] Metal oxo species have been found capable of catalytic, including asymmetric oxidations of various types. Some metal-oxo complexes can activate C-H bonds to give an aldehyde or alcohol.[4] Some metal oxides have been used to catalyze reduction of organic compounds.[5]
-
 Selection of molecular metal oxides. From right, vanadyl chloride (d0), a tungsten oxo carbonyl (d2), permanganate (d0), [ReO2(pyridine)4]+ (d2), simplified view of compound I (a state of cytochrome P450, d4), and trismesityliridium oxide (d4).
Selection of molecular metal oxides. From right, vanadyl chloride (d0), a tungsten oxo carbonyl (d2), permanganate (d0), [ReO2(pyridine)4]+ (d2), simplified view of compound I (a state of cytochrome P450, d4), and trismesityliridium oxide (d4).
Metalloenzymes
Iron(IV)-oxo species
Iron(IV)-oxo compounds are intermediates in many oxidations catalysed by heme-containing enzymes. One of the most widely studied examples is cytochrome p450 enzymes, which use a heme cofactor and commonly oxidize an alkyl group to an alcohol, a very difficult oxidation to do synthetically. Similarly, methane monooxygenase (MMO) oxidizes methane to methanol via oxygen atom transfer from an iron-oxo intermediate at its non-heme di-iron center. First, C-H bonds are quite resistant to oxidation and are generally unreactive at moderate temperatures (see C-H bond activation). Second, harsh oxidizing agents will generally oxidize an alcohol to a carboxylic acid, but these enzymes are able to oxidize an alkyl group to an alcohol without further oxidation to a carbonyl or carboxylic acid. The oxidant used in these enzymatic reactions is molecular oxygen in contrast with the harsh, toxic chemicals often found in conventional synthetic organic oxidations.[6] As is generally the case with enzymatic reactions, these oxidations are chemically selective and take place at fast rates in aqueous solvent. Much of the effort in producing synthetic C-H bond activation catalysts has been inspired by these well designed natural catalysts.[4]
Molybdenum/tungsten oxo species
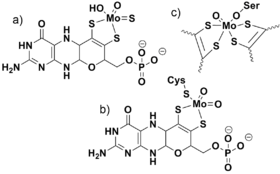 Fig. 4 The three structural families of molybdenum cofactors: a) Xanthine oxidase, b) Sulfite oxidase, and c) (DMSO) reductase. The DMSO reductase structure contains two molybdopterin ligands. They are omitted from the figure for simplicity. The rest of the heterocycle is similar to what is shown for the other two cofactors.
Fig. 4 The three structural families of molybdenum cofactors: a) Xanthine oxidase, b) Sulfite oxidase, and c) (DMSO) reductase. The DMSO reductase structure contains two molybdopterin ligands. They are omitted from the figure for simplicity. The rest of the heterocycle is similar to what is shown for the other two cofactors.
The oxo ligand (or analagous sulfido ligand) is nearly ubiquitous in molybdenum and tungsten chemistry, appearing in the ores containing these elements, throughout their synthetic chemistry, and also in their biological use. The biologically transported species and starting point for biosynthesis is generally accepted to be oxometallates MoO4−2 or WO4−2. All Mo/W enzymes except nitrogenase have the molybdopterin prosthetic group which generally cycles between Mo(IV) and Mo(VI) in one electron steps. Though there is some variation among these enzymes, members from all three families involve oxygen atom transfer between the Mo center and the substrate.[7] Representative reactions from each of the three structural classes are:
- SO3−2 + H2O → SO4−2 + 2H+ + 2e- - (Sulfite oxidase)
- H3CS(O)CH3 (DMSO) + 2H+ + 2e- → H3CSCH3 (DMS) + H2O - (DMSO reductase)
The three different classes of molybdenum cofactors are shown in the Figure. The biological use of tungsten mirrors that of molybdenum.[8]
Oxygen-evolving complex
The active site for the oxygen-evolving complex (OEC) of Photosystem II (PSII) is a Mn4OxCa centre with several bridging oxo ligands that participate in the oxidation of water to molecular oxygen. The OEC is proposed to utilize a terminal oxo intermediate as a part of the water oxidation reaction.[9] This complex is responsible for the production of nearly all of earth's molecular oxygen. This key link in the oxygen cycle is necessary for much of the biodiversity present on earth.
The "oxo wall"
The term "oxo wall" is jargon used to describe the fact that no molecular oxo complexes is known for octahedral metal centers with d-electron counts beyond 4. A correlary is that the reactivity of complexes containing a terminal oxo ligand depends on the metal d-electron count. For octahedral d4 oxo complexes, the oxo ligand is nucleophilic. When the d-electron count is lower than 4, the oxo ligands become electrophilic. Oxo compounds for the vanadium through iron triads (groups 3-8) are well known, whereas terminal oxo compounds for metals in the cobalt through zinc triads (groups 9-12) are rare and invariably feature metals with coordination numbers lower than 6. This trend holds for other metal-ligand multiple bonds.
Terminal oxo ligands are also rather rare for the titanium triad, especially zirconium and hafnium and is unknown for group 3 metals (scandium, yttrium, and lanthanum).[1]
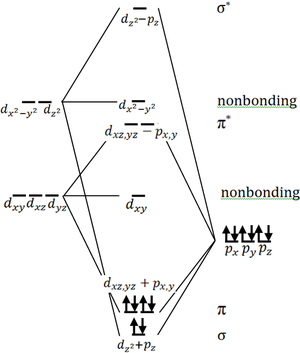
Bonding in terminal metal oxides
Terminal oxo ligation is stable for low d electron counts, but the stable oxidation states of late transition metals do not have low enough d counts to stabilize terminal oxo formation.[10] The higher d counts of late transition metals lead to electrons in anti-bonding molecular orbitals and destabilization of the metal-oxygen multipl bond (see Fig. 2). Generally, very high oxidation states are destabilized by electrostatics: a buildup of localized charge is always very destabilizing. Metals such as the group 6 metals (Cr, Mo, and W), which are very often found in the high +6 oxidation state, are stabilized by orbital considerations. The +6 oxidation state here yields a noble gas electron configuration, which is very stable from an electron orbital point of view, thus offsetting destabilizing electrostatic effects.
The d orbitals for the very early transition are quite high energy and diffuse. Thus they do not overlap well in energy with the oxygen p orbitals, and in a metal-oxo interaction, the electron density tends to remain more localized on the oxygen in a more ionic type interaction. This makes the oxygen very nucleophilic and likely to attack other metal centers to yield a bridging configuration to delocalize the charge over multiple metals.
M=O groups with coordination numbers <6
The iridium oxo complex Ir(O)(mesityl)3 poses an apparent exception to the oxo-wall, but in this case, iridium is tetrahedral.[11] Similarly with platinum-,[12] palladium-,[13] and gold-oxo[14] feature square planar metals. These complexes have M=O double bonds.[12]
See also
- metal-ligand multiple bond
- oxide
- polyoxometalate
- metallate
- oxophilic
- dioxygen complex
References
- ^ a b c d Nugent, W. A., Mayer, J. M. "Metal-Ligand Multiple Bonds." John Wiley & Sons, New York, 1988.
- ^ Sharpless, K.B.; Flood, T.C. (1971). "Oxotransition metal oxidants as mimics for the action of mixed-function oxygenases. 'NIH shift' with chromyl reagents". J. Am. Chem. Soc. 93 (9): 2316–8. doi:10.1021/ja00738a039. PMID 5553075.
- ^ Holm, R. H. (1987). "Metal-centered oxygen atom transfer reactions". Chem. Rev. 87 (6): 1401–1449. doi:10.1021/cr00082a005.
- ^ a b Gunay A. and Theopold, K.H. (2010). "C-H Bond Activations by Metal Oxo Compounds". Chem. Rev. 110 (2): 1060–1081. doi:10.1021/cr900269x.
- ^ Du, G. and Abu-Omar, M.M. (2008). "Oxo and Imido Complexes of Rhenium and Molybdenum in Catalytic Reductions". Current Organic Chemistry 12 (14): 1185–1198. doi:10.2174/138527208785740238.
- ^ Brunold, T.C. (2007). "Synthetic iron-oxo ‘diamond core’ mimics structure of key intermediate in methane monooxygenase catalytic cycle". Proc. Natl. Acad. Sci. U.S.A. 104 (52): 20641–20642. doi:10.1073/pnas.0710734105.
- ^ Schwarz, G., Mendel, R.R., and Ribbe, M.W. (2009). "Molybdenum cofactors, enzymes and pathways". Nature 460 (7257): 839–847. doi:10.1038/nature08302.
- ^ Mukund, S. and Adams, M.W.W. (1996). "Molybdenum and Vanadium Do Not Replace Tungsten in the Catalytically Active Forms of the Three Tungstoenzymes in the Hyperthermophilic Archaeon Pyrococcus furiosus". J. Bact.: 163–167.
- ^ Yasufumi Umena, Keisuke Kawakami, Jian-Ren Shen, and Nobuo Kamiya "Crystal structure of oxygen-evolving photosystem II at a resolution of 1.9 Å" Nature 2011, vol. 473, 55-60 doi:10.1038/nature09913
- ^ Cao, Rui et al. (2008). "Late Transition Metal-Oxo Compounds and Open-Framework Materials that Catalyze Aerobic Oxidations". Adv. Inorg. Chem. 60: 245–269. doi:10.1016/S0898-8838(08)00006-8..
- ^ Hay-Motherwell, R. S.; Wilkinson, G.; Hussain-Bates, B.; Hursthouse, M. B. (1993). "Synthesis and X-ray Crystal Structure of Oxotrimesityl-Iridium(V)". Polyhedron 12 (16): 2009–2012. doi:10.1016/S0277-5387(00)81474-6.
- ^ a b Anderson, T. M. et al. (2004). "A Late-Transition Metal Oxo Complex: K7Na9[O=PtIV(H2O)L2], L = [PW9O<sub34]9-". Science 306 (5704): 2074–2077. doi:10.1126/science.1104696.
- ^ Anderson, T. M. et al. (2005). "A Palladium-Oxo Complex. Stabilization of This Proposed Catalytic Intermediate by an Encapsulating Polytungstate Ligand". J. Am. Chem. Soc. 127 (34): 11948–11949. doi:10.1021/ja054131h.
- ^ Cao, R. et al.. "Terminal Gold-Oxo Complexes". J. Am. Chem. Soc. 130: 2877–2877.
Categories:- Ligands
- Oxides
-
Wikimedia Foundation. 2010.