- Tetrachloroethylene
-
Tetrachloroethylene 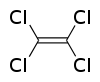
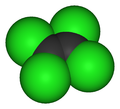 TetrachloroetheneOther namesPerchloroethene; Perchloroethylene; Perc; PCE
TetrachloroetheneOther namesPerchloroethene; Perchloroethylene; Perc; PCEIdentifiers CAS number 127-18-4 
ChemSpider 13837281 
UNII TJ904HH8SN 
EC number 204-825-9 UN number 1897 KEGG C06789 
ChEBI CHEBI:17300 
ChEMBL CHEMBL114062 
RTECS number KX3850000 Jmol-3D images Image 1 - Cl/C(Cl)=C(/Cl)Cl
Properties Molecular formula C2Cl4 Molar mass 165.83 g mol−1 Appearance Clear, colorless liquid Density 1.622 g/cm3 Melting point -19 °C, 254 K, -2 °F
Boiling point 121.1 °C, 394 K, 250 °F
Solubility in water 0.015 g/100 mL (20 °C) Viscosity 0.89 cP at 25 °C Hazards MSDS External MSDS R-phrases R40 R51/53 S-phrases S23 S36/37 S61 Main hazards Harmful (Xn),
Dangerous for
the environment (N)NFPA 704 Flash point Not flammable Related compounds Related Related organohalides Tetrabromoethylene
TetraiodoethyleneRelated compounds Trichloroethylene
Dichloroethene
TetrachloroethaneSupplementary data page Structure and
propertiesn, εr, etc. Thermodynamic
dataPhase behaviour
Solid, liquid, gasSpectral data UV, IR, NMR, MS  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Tetrachloroethylene, also known under its systematic name tetrachloroethene and many other names, is a chlorocarbon with the formula Cl2C=CCl2. It is a colourless liquid widely used for dry cleaning of fabrics, hence it is sometimes called "dry-cleaning fluid." It has a sweet odor detectable by most people at a concentration of 1 part per million (1 ppm). Worldwide production was about 1 megatonne in 1985.[1]
Contents
Production
Michael Faraday first synthesized tetrachloroethene in 1821 by thermal decomposition of hexachloroethane.
- C2Cl6 → C2Cl4 + Cl2
Most tetrachloroethene is produced by high temperature chlorinolysis of light hydrocarbons. The method is related to Faraday's discovery since hexachloroethane is generated and thermally decomposes.[1] Side products include carbon tetrachloride, hydrogen chloride, and hexachlorobutadiene.
Several other methods have been developed. When 1,2-dichloroethane is heated to 400 °C with chlorine, tetrachloroethene is produced by the chemical reaction:
- ClCH2CH2Cl + 3 Cl2 → Cl2C=CCl2 + 4 HCl
This reaction can be catalyzed by a mixture of potassium chloride and aluminium chloride or by activated carbon. Trichloroethylene is a major byproduct, which is separated by distillation.
According to an EPA report of 1976, the quantity of Tetrachloroethylene (also known as perchloroethylene or PCE) produced in the United States in just one year 1973, totaled 706 million pounds (320,000 metric tons). Diamond Shamrock, Dow Chemical Company, E.I DuPont and Vulcan Materials Company (Chemical Division) were among the top eight producers nationwide. [2]
Uses
Tetrachloroethylene is an excellent solvent for organic materials. Otherwise it is volatile, highly stable, and nonflammable. For these reasons, it is widely used in dry cleaning. Usually as a mixture with other chlorocarbons, it is also used to degrease metal parts in the automotive and other metalworking industries. It appears in a few consumer products including paint strippers and spot removers.
Historical applications
Tetrachloroethene was once extensively used as an intermediate in the manufacture of HFC-134a and related refrigerants. In the early 20th century, tetrachloroethene was used for the treatment for hookworm infestation.[3]
Health and safety
The International Agency for Research on Cancer has classified tetrachloroethene as a Group 2A carcinogen, which means that it is probably carcinogenic to humans.[4] Like many chlorinated hydrocarbons, tetrachloroethene is a central nervous system depressant and can enter the body through respiratory or dermal exposure.[5] Tetrachloroethene dissolves fats from the skin, potentially resulting in skin irritation.
Animal studies and a study of 99 twins by Dr. Samuel Goldman and researchers at the Parkinson's Institute in Sunnyvale, California determined there is a "lot of circumstantial evidence" that exposure to Tetrachloroethlene increases the risk of developing Parkinson's disease ninefold. Larger population studies are planned.[6]
At temperatures over 600 °F (316 °C), such as in welding, tetrachloroethylene can decompose into phosgene, an extremely poisonous gas.[7][8] Tetrachloroethylene should not be used near welding operations, flames, or hot surfaces.[9]
Testing for exposure
Tetrachloroethene exposure can be evaluated by a breath test, analogous to breath-alcohol measurements. Because it is stored in the body's fat and slowly released into the bloodstream, tetrachloroethene can be detected in the breath for weeks following a heavy exposure. Tetrachloroethylene and trichloroacetic acid (TCA), a breakdown product of tetrachloroethene, can be detected in the blood.
In Europe, the Scientific Committee on Occupational Exposure Limits (SCOEL) recommends for tetrachloroethylene an occupational exposure limit (8h time-weighted average) of 20 ppm and a short-term exposure limit (15 min) of 40 ppm.[10]
Environmental contamination
Tetrachloroethene is a common soil contaminant. With a specific gravity greater than 1, tetrachloroethylene will be present as a dense nonaqueous phase liquid if sufficient quantities of liquid are spilled in the environment. Because of its mobility in groundwater, its toxicity at low levels, and its density (which causes it to sink below the water table), cleanup activities are more difficult than for oil spills. Recent research has focused on the in place remediation of soil and ground water pollution by tetrachloroethylene. Instead of excavation or extraction for above-ground treatment or disposal, tetrachloroethylene contamination has been successfully remediated by chemical treatment or bioremediation. Bioremediation has been successful under anaerobic conditions by reductive dechlorination by Dehalococcoides sp. and under aerobic conditions by cometabolism by Pseudomonas sp.[11][12] Partial degradation daughter products include trichloroethylene, cis-1,2-dichloroethene and vinyl chloride; full degradation converts tetrachloroethylene to ethene and chloride dissolved in water.
It has been estimated that about 85% of tetrachloroethylene is released into the atmosphere; OECD models assumed 90% release into the air and 10% to water. Based on these models, its distribution in the environment is estimated to be in the air (76.39% - 99.69%), water (0.23% - 23.2%), soil (0.06-7%), with the remainder in the sediment and biota. Estimates of lifetime in the atmosphere vary, but a 1987 survey estimated the lifetime in the air has been estimated at about 2 months in the Southern Hemisphere and 5–6 months in the Northern Hemisphere. Degradation products observed in a laboratory include phosgene, trichloroacetyl chloride, hydrogen chloride, carbon dioxide, and carbon monoxide. In water, tetrachloroethylene is degraded very slowly by hydrolysis, and it is persistent under aerobic conditions. It is degraded through reductive dechlorination under anaerobic conditions, with the degradation products including trichloroethene, dichloroethene, vinyl chloride, ethene, and ethane.[13]
References
- ^ a b M. Rossberg et al. “Chlorinated Hydrocarbons” in Ullmann’s Encyclopedia of Industrial Chemistry, 2006, Wiley-VCH, Weinheim. doi:10.1002/14356007.a06_233.pub2
- ^ "Assessment of Hazardous Waste Practices: Organic Chemicals, Pesticides and Explosives Industries" prebpublication issue for EPA Libraries and Solid Waste Management Agencies under contract # 68-01-2919, USEPA 1976
- ^ Young, M.D.; et al. (1960). "The Comparative Efficacy of Bephenium Hydroxynaphthoate and Tetrachloroethylene against Hookworm and other Parasites of Man". American Journal of Tropical Medicine and Hygiene 9 (5): 488–491. PMID 13787477.
- ^ IARC monograph. Tetrachloroethylene, Vol. 63, p. 159. Last Updated May 20, 1997. Last retrieved June 22, 2007.
- ^ Control of Exposure to Perchloroethylene in Commercial Drycleaning. Hazard Controls: Publication 97-157. National Institute for Occupational Safety and Health.
- ^ Industrial Solvent Linked to Increased Risk of Parkinson's Disease
- ^ Medical Management Guidelines for Tetrachloroethylene
- ^ Common cleaners can turn into poison gas
- ^ Working safely with tetrachloroethylene
- ^ "SCOEL recommendations". 2011-04-22. http://ec.europa.eu/social/keyDocuments.jsp?type=0&policyArea=82&subCategory=153&country=0&year=0&advSearchKey=recommendation&mode=advancedSubmit&langId=en. Retrieved 2011-04-22.
- ^ Ryoo, D., Shim, H., Arenghi, F. L. G., Barbieri, P., Wood T. K. (2001). "Tetrachloroethylene, Trichloroethylene, and Chlorinated Phenols Induce Toluene-o-xylene Monooxoygenase Activity in Pseudomonas Stutzeri OX1". Applied Microbiol Biotechnol 56 (3–4): 545–549. doi:10.1007/s002530100675.
- ^ Deckard, L. A., Wills, J. C., Rivers, D. B. (1994). "Evidence for aerobic degradation of tetrachloroethylene by bacterial isolate". Biotechnol. Lett. 16 (11): 1221–1224. doi:10.1007/BF01020855.
- ^ Watts P. (2006). Concise International Chemical Assessment Document 68: TETRACHLOROETHENE, World Health Organization
Further reading
- "Toxicological Profile for Tetrachloroethene". Agency for Toxic Substances and Disease Registry. 1997. http://www.atsdr.cdc.gov/toxprofiles/tp.asp?id=265&tid=48.
- Doherty, R.E. (2000). "A History of the Production and Use of Carbon Tetrachloride, Tetrachloroethylene, Trichloroethylene and 1,1,1-Trichloroethane in the United States: Part 1 - Historical Background; Carbon Tetrachloride and Tetrachloroethylene". Environmental Forensics 1 (2): 69–81. doi:10.1006/enfo.2000.0010.
External links
- ATSDR Case Studies in Environmental Medicine: Tetrachloroethylene Toxicity U.S. Department of Health and Human Services
- Australian National Pollutant Inventory (NPI) page
- "Toxic Fumes May Have Made Gunman Snap", by Julian Kesner, New York Daily News, April 20, 2007.
Categories:- Organochlorides
- Halogenated solvents
- Hazardous air pollutants
- IARC Group 2A carcinogens
Wikimedia Foundation. 2010.

