- 1,2-Dichloroethene
-
cis-1,2-Dichloroethene (Z) (left) and trans-1,2-Dichloroethene (E) (right) 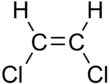
 1,2-DichloroetheneOther names1,2-Dichloroethylene
1,2-DichloroetheneOther names1,2-Dichloroethylene
1,2-DCEIdentifiers CAS number 156-59-2 (Z)  , 156-60-5 (E), 540-59-0 (racemate)
, 156-60-5 (E), 540-59-0 (racemate)ChemSpider 10438 
KEGG C06792 
ChEBI CHEBI:18882 
Jmol-3D images Image 1
Image 2- Cl[C@H]=CCl
ClC=CCl
Properties Molecular formula C2H2Cl2 Molar mass 96.95 g/mol Density Z: 1.28 g/cm³
E: 1.26 g/cm³Melting point Z: -81 °C
E: -81 °CBoiling point Z: 60.3 °C
E: 47.5 °CDipole moment Z (cis): 1.9 D
E (trans): 0 D (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references 1,2-Dichloroethene, commonly called 1,2-dichloroethylene or 1,2-DCE, is an organochloride with the molecular formula C2H2Cl2. It is a highly flammable, colorless liquid with a sharp, harsh odor. It can exist as either of two geometric isomers, cis-1,2-dichloroethene or trans-1,2-dichloroethene, but is often used as a mixture of the two. It is minimally soluble (5090 mg/L for the cis-isomer[1]) in water, and soluble in ethanol, diethyl ether, acetone, benzene, and chloroform.
1,2-DCE is used as a solvent for waxes, resins, polymers, fats, and lacquers. It is also used as an intermediate in the preparation of other chlorinated solvents.
The major health effect of inhalation of vapors of 1,2-DCE is narcosis; it has been used in a combination with diethyl ether as an anesthetic. In high concentrations, exposure to 1,2-DCE causes central nervous system depression; in milder exposures, it can produce nausea, vomiting, weakness, tremor, epigastric cramps, burning of the eyes and vertigo.
See also
References
- ^ Schwarzenbach et al. (2003) Environmental Organic Chemistry, Pub. Wiley Interscience
External links
Categories:- Organochlorides
- Alkenes
- Halogenated solvents
- Cl[C@H]=CCl
Wikimedia Foundation. 2010.
