- Hox gene
-
Hox genes are a group of related genes that determine the basic structure and orientation of an organism.[1]
Hox genes are critical for the proper placement of segment structures of animals during early embryonic development (e.g. legs, antennae, and wings in fruit flies or the different vertebrate ribs in humans).
Hox genes are defined as having -
- a DNA sequence known as the homeobox
- their location in gene clusters on the genome
- an expression pattern along the cephalo-caudal (head to tail) axis that corresponds to the relative location of their genes within the Hox gene cluster.[2]
The homeobox
The homeobox is a 180 nucleotide long DNA sequence that encodes a 60 amino acid long protein domain known as the homeodomain.
Hox proteins
The products of Hox genes are known as Hox proteins. Hox proteins are transcription factors, as they are capable of binding to specific nucleotide sequences on the DNA called enhancers where they either activate or repress genes. The same Hox protein can act as a repressor at one gene and an activator at another. For example, in flies (Drosophila melanogaster) the protein product of the Hox gene Antennapedia activates genes that specify the structures of the 2nd thoracic segment, which contains a leg and a wing, and represses genes involved in eye and antenna formation.[3] Thus, legs and wings, but not eyes and antennae, will form wherever the Antennapedia protein is located. The ability of Hox proteins to bind DNA is conferred by a part of the protein referred to as the homeodomain.
The homeodomain
The homeodomain is a 60 amino acid long DNA-binding domain (encoded by the homeobox on the DNA). This amino acid sequence folds into a helix-turn-helix motif that is stabilized by a third helix.
The consensus polypeptide chain is (typical intron position noted with dashes):[4]
RRRKRTA-YTRYQLLE-LEKEFLF-NRYLTRRRRIELAHSL-NLTERHIKIWFQN-RRMK-WKKEN
The first genes found to encode homeodomain proteins were Drosophila developmental control genes, in particular Hom-C genes, from which the name homeobox was derived. However, many homeobox genes are not homeotic genes; the homeobox is a sequence motif, while "homeotic" is a functional description for genes that scaffold structures or developmental patterns.[5]
Sequence conservation
The homeodomain protein motif is highly conserved across vast evolutionary distances. In addition, homeodomains of individual Hox proteins usually exhibit greater similarity to homeodomains in other species than to proteins encoded by adjacent genes within their own Hox cluster. These two observations led to the suggestions that Hox gene clusters evolved from a single Hox gene via tandem duplication and subsequent divergence and that a prototypic Hox gene cluster containing at least seven different Hox genes was present in the common ancestor of all bilaterian animals.[6]
Classification of Hox proteins
Proteins with high degree of sequence similarity are also generally assumed to exhibit a high degree of functional similarity, e.g. Hox proteins with identical homeodomains are assumed to have identical DNA-binding properties (unless additional sequences are known to influence that). To identify the set of proteins between two different species that are most likely to be most similar in function, classification schemes are used. For Hox proteins, three different classification schemes exist: phylogenetic inference based, synenty based, and sequence similarity based.[7]
The functional equivalence of such a set of Hox proteins can be demonstrated by the fact that a fly can function perfectly well with a chicken Hox protein in place of its own.[8] This means that, despite having a last common ancestor that lived over 670 million years ago,[9] a given Hox protein in chickens and the homologous gene in flies are so similar that they can actually take each other's places.
Genes regulated by Hox proteins
Hox genes act at many levels within developmental gene hierarchies: at the "executive" level they regulate genes that in turn regulate large networks of other genes (like the gene pathway that forms an appendage). They also directly regulate what are called realisator genes or effector genes that act at the bottom of such hierarchies to ultimately form the tissues, structures, and organs of each segment. Segmentation involves such processes as morphogenesis (differentiation of precursor cells into their terminal specialized cells), the tight association of groups of cells with similar fates, the sculpting of structures and segment boundaries via programmed cell death, and the movement of cells from where they are first born to where they will ultimately function, so it is not surprising that the target genes of Hox genes promote cell division, cell adhesion, apoptosis, and cell migration.[10]
Examples of targets Organism Target gene Normal function of target gene Regulated by Drosophila distal-less activates gene pathway for limb formation ULTRABITHORAX[11] (represses distal-less)
distal-less activates gene pathway for limb formation ABDOMINAL-A[11] (represses distal-less)
decapentaplegic triggers cell shape changes in the gut that are required for normal visceral morphology
ULTRABITHORAX[12] (activates decapentaplegic)
reaper Apoptosis: localized cell death creates the segmental boundary between the maxilla and mandible of the head
DEFORMED[13] (activates reaper)
dapentaplegic prevents the above cell changes in more posterior positions
ABDOMINAL-B[12] (repress decapentaplegic)
Mouse EphA7 Cell adhesion: causes tight association of cells in distal limb that will form digit, carpal and tarsal bones
HOX-A13[10] (activates EphA7)
Cdkn1a Cell cycle: differentiation of myelomonocyte cells into monocytes (white blood cells), with cell cycle arrest
Hox-A10[14] (activates Cdkn1a)
Enhancer sequences that are bound by homeodomains
The DNA sequence that is bound by the homeodomain protein contains the nucleotide sequence TAAT, with the 5' terminal T being the most important for binding.[15] This sequence is conserved in nearly all sites recognized by homeodomains, and probably distinguishes such locations as DNA binding sites. The base pairs following this initial sequence are used to distinguish between homeodomain proteins, all of which have similar recognition sites. For instance, the nucleotide following the TAAT sequence is recognized by the amino acid at position 9 of the homeodomain protein. In the maternal protein Bicoid, this position is occupied by lysine, which recognizes and binds to the nucleotide guanine. In Antennapedia, this position is occupied by glutamine, which recognizes and binds to adenine. If the lysine in Bicoid is replaced by glutamine, the resulting protein will recognize Antennapedia-binding enhancer sites.[16]
Regulation of Hox genes
Just as Hox genes regulate realisator genes, they are in turn regulated themselves by gap genes and pair-rule genes, which are in their turn regulated by maternally-supplied mRNA. This results in a transcription factor cascade: maternal factors activate gap or pair-rule genes; gap and pair-rule genes activate Hox genes; then, finally, Hox genes activate realisator genes that cause the segments in the developing embryo to differentiate. Regulation is achieved via protein concentration gradients, called morphogenic fields. For example, high concentrations of one maternal protein and low concentrations of others will turn on a specific set of gap or pair-rule genes. In flies, stripe 2 in the embryo is activated by the maternal proteins Bicoid and Hunchback, but repressed by the gap proteins Giant and Kruppel. Thus, stripe 2 will only form wherever there is Bicoid and Hunchback, but not where there is Giant and Kruppel.[17]
MicroRNA strands located in hox clusters have been shown to inhibit more anterior hox genes ("posterior prevalence phenomenon"), possibly to better fine tune its expression pattern.[18]
Non-coding RNA (ncRNA) has been shown to be abundant in Hox clusters. In humans, 231 ncRNA may be present. One of these, HOTAIR, silences in trans (it is transcribed from the HOXC cluster and inhibits late HOXD genes) by binding to Polycomb-group proteins (PRC2).[19]
The chromatin structure is essential for transcription but it also requires the cluster to loop out of the chromosomal territory.[20]
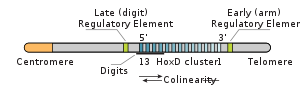
In higher animals and humans, retinoic acid regulates differential expression of Hox genes along the anteroposterior axis.[21] Genes in the 3' ends of Hox clusters are induced by retinoic acid resulting in expression domains that extend more anteriorly in the body compared to 5' Hox genes that are not induced by retinoic acid resulting in expression domains that remain more posterior.
Quantitative PCR has shown several trends regarding colinearity: the system is in equlibrium and the total number of transcripts depends on the number of genes present according to a linear relationship.[22]
Homeotic mutations
Incorrect expression of Hox genes can lead to major changes in the morphology of the individual. Homeotic mutations were first identified in 1894, when William Bateson noticed that floral stamens occasionally appeared in the wrong place; he found four example flowers in which the stamens would grow in the place where petals normally grow.
In the late 1940s, Edward Lewis began studying homeotic mutation on Drosophila melanogaster which caused bizarre rearrangements of body parts. Mutations in the genes that code for limb development can cause deformity or lead to death. For an example, mutations in the Antennapedia gene cause legs instead of the antenna to develop on the head of a fly.[23]
Another famous example in the Drosophila melanogaster is the mutation of the Ultrabithorax Hox gene, which specifies the 3rd thoracic segment. Normally, this segment displays a pair of legs and a pair of halteres (a reduced pair of wings used for balancing). In the mutant lacking functional Ultrabithorax protein, the 3rd thoracic segment now expresses the same structures found on the segment to its immediate anterior, the 2nd thoracic segment, which contains a pair of legs and a pair of (fully developed) wings. These mutants sometimes occur in wild populations of flies, and it was these mutants that led to the discovery of Hox genes.
Colinearity of Hox genes
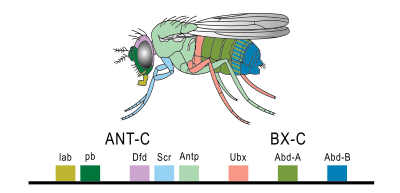
In some organisms, especially vertebrates, the various Hox genes are situated very close to one another on the chromosome in groups or clusters. Interestingly, the order of the genes on the chromosome is the same as the expression of the genes in the developing embryo, with the first gene being expressed in the anterior end of the developing organism. The reason for this colinearity is not yet completely understood. The diagram above shows the relationship between the genes and protein expression in flies.
Hox nomenclature
Hox genes in different phyla have been given different names, which has led to confusion about nomenclature. The complement of Hox genes of the Ecdysozoa (arthropods, nematodes, and so on) is made up of two clusters, the Antennapedia complex and the Bithorax complex, which together are referred to as the HOM-C (for Homeotic Complex). Hox genes in deuterostomes (echinoderms, chordates) are correctly referred to as Hox genes, and are arranged in four clusters: Hoxa, Hoxb, Hoxc, and Hoxd. Although it is technically incorrect to refer to homeotic genes in non-deuterostome phyla as "Hox genes"[citation needed], the practice of using "Hox" in place of "Hom-C" is now acceptable even in the scientific literature.
Human genes
Humans contain Hox genes in four clusters:
cluster chromosome genes HOXA chromosome 7 HOXA1, HOXA2, HOXA3, HOXA4, HOXA5, HOXA6, HOXA7, HOXA9, HOXA10, HOXA11, HOXA13 HOXB chromosome 17 HOXB1, HOXB2, HOXB3, HOXB4, HOXB5, HOXB6, HOXB7, HOXB8, HOXB9, HOXB13 HOXC chromosome 12 HOXC4, HOXC5, HOXC6, HOXC8, HOXC9, HOXC10, HOXC11, HOXC12, HOXC13 HOXD chromosome 2 HOXD1, HOXD3, HOXD4, HOXD8, HOXD9, HOXD10, HOXD11, HOXD12, HOXD13 History
Christiane Nüsslein-Volhard and Eric F. Wieschaus identified and classified 15 genes of key importance in determining the body plan and the formation of body segments of the fruit fly Drosophila melanogaster. Edward B. Lewis studied the next step - Hox genes that govern the development of a larval segment into a specific body segment. Homeotic means that something has been changed into the likeness of something else. Lewis found a colinearity in time and space between the order of the genes in the bithorax complex and their affected regions in the segments. For their work they were awarded the Nobel Prize in Physiology or Medicine in 1995.
Further information: Nobel foundation websiteSee also
References
- ^ Dictionary.com - Hox gene [1]. Accessed March 31, 2011.
- ^ Carroll S. B. (1995). "Homeotic genes and the evolution of arthropods and chordates". Nature 376 (6540): 479–85. doi:10.1038/376479a0. PMID 7637779.
- ^ Cesares and Mann 1998; Plaza et al. 2001
- ^ http://www.csb.ki.se/groups/tbu/homeo/consensus.gif
- ^ Burglin, T.(2005). The Homeobox Page. http://www.cbt.ki.se/groups/tbu/homeo.html#Structure%20of%20the%20homeodomain
- ^ McGinnis W.; R. Krumlauf (1992). "Homeobox genes and axial patterning". Cell 68 (2): 283–302. doi:10.1016/0092-8674(92)90471-N. PMID 1346368.
- ^ Hueber S.D.; Weiller G.F., Djordjevic, M. A, Frickey, T. (2010). "Improving Hox Protein Classification across the Major Model Organisms". PLoS One: e10820. doi:10.1371/journal.pone.0010820. PMC 2876039. PMID 20520839. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2876039.
- ^ Lutz, B.; H.C. Lu, G. Eichele, D. Miller, and T.C. Kaufman (1996). "Rescue of Drosophila labial null mutant by the chicken ortholog Hoxb-1 demonstrates that the function of Hox genes is phylogenetically conserved". Genes & Development 10 (2): 176–184. doi:10.1101/gad.10.2.176. PMID 8566751.
- ^ Ayala, F.J.; A. Rzhetskydagger (20 January 1998). "Origin of the metazoan phyla: Molecular clocks confirm paleontological estimates". Proc Natl Acad Sci 95 (2): 606–11. doi:10.1073/pnas.95.2.606. PMC 18467. PMID 9435239. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=18467.
- ^ a b Pearson, JC, Lemons, D. and McGinnis, W. Modulating Hox gene functions during animal body patterning. Nature Rev. Genet. 6, 893–904 (2005).
- ^ a b Vachon, G. et al. Homeotic genes of the bithorax complex repress limb development in the abdomen of the Drosophila embryo through the target gene Distal-less. Cell 71, 437–450 (1992).
- ^ a b Capovilla, M. & Botas, J. Functional dominance among Hox genes: repression dominates activation in the regulation of dpp. Development 125, 4949–4957 (1998).
- ^ Lohmann, I., McGinnis, N., Bodmer, M. & McGinnis, W. The Drosophila Hox gene Deformed sculpts head morphology via direct regulation of the apoptosis activator reaper. Cell 110, 457–466 (2002).
- ^ Bromleigh, V. C. & Freedman, L. P. p21 is a transcriptional target of HOXA10 in differentiating myelomonocytic cells. Genes Dev. 14, 2581–2586 (2000).
- ^ Gilbert, Developmental Biology, 2006
- ^ Hanes and Brent 1989, 1991
- ^ Small S, 1992. Regulation of even-skipped stripe 2 in the Drosophila embryo. EMBO J. 1992 Nov;11(11):4047-57
- ^ Lempradl A, Ringrose L. 2008 How does noncoding transcription regulate Hox genes? Bioessays. 30(2):110-21.
- ^ Rinn, JL et al.; Kertesz, M; Wang, JK; Squazzo, SL; Xu, X; Brugmann, SA; Goodnough, LH; Helms, JA et al. (2007). "Functional Demarcation of Active and Silent Chromatin Domains in Human HOX Loci by Non-Coding RNAs". Cell 129 (7): 1311–23. doi:10.1016/j.cell.2007.05.022. PMC 2084369. PMID 17604720. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2084369.
- ^ Fraser, P; Bickmore, W. (2007). "Nuclear organization of the genome and the potential for gene regulation". Nature 447 (7143): 413–7. doi:10.1038/nature05916. PMID 17522674.
- ^ Duester, G (September 2008). "Retinoic Acid Synthesis and Signaling during Early Organogenesis". Cell 134 (6): 921–31. doi:10.1016/j.cell.2008.09.002. PMC 2632951. PMID 18805086. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2632951.
- ^ Montavon, et al.; Le Garrec, JF; Kerszberg, M; Duboule, D (2008). "Modeling Hox gene regulation in digits: reverse collinearity and the molecular origin of thumbness". Genes Dev. 22 (3): 346–59. doi:10.1101/gad.1631708. PMC 2216694. PMID 18245448. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2216694.
- ^ Pierce, Benjamin A. Genetics:A Conceptual approach.2nd edition
Further reading
- Hunt, Paul (1998). "The Function of Hox Genes". In Bittar, E. Edward. Developmental biology. Elsevier. ISBN 9781559388160. http://books.google.com/books?id=I4EAA453yjMC&pg=PA261.
External links
- The Homeotic Selector Genes in Developmental Biology, 6th Edition by Scott F. Gilbert (2000) Published by Sinauer Associates, Inc. ISBN 0-87893-243-7.
Categories:- Developmental genes and proteins
Wikimedia Foundation. 2010.