- NEU3
-
Sialidase 3 (membrane sialidase) Identifiers Symbols NEU3; FLJ12388; SIAL3 External IDs OMIM: 604617 MGI: 1355305 HomoloGene: 4847 GeneCards: NEU3 Gene EC number 3.2.1.18 Gene Ontology Molecular function • catalytic activity
• exo-alpha-sialidase activity
• hydrolase activity, acting on glycosyl bonds
• alpha-sialidase activityCellular component • plasma membrane
• integral to plasma membraneBiological process • carbohydrate metabolic process
• ganglioside catabolic processSources: Amigo / QuickGO RNA expression pattern 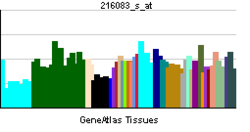
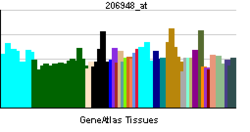
More reference expression data Orthologs Species Human Mouse Entrez 10825 50877 Ensembl ENSG00000162139 ENSMUSG00000035239 UniProt Q9UQ49 Q543I9 RefSeq (mRNA) NM_006656 NM_016720.2 RefSeq (protein) NP_006647 NP_057929.1 Location (UCSC) Chr 11:
74.7 – 74.73 MbChr 7:
106.96 – 106.98 MbPubMed search [1] [2] Sialidase-3 is an enzyme that in humans is encoded by the NEU3 gene.[1][2]
This gene product belongs to a family of glycohydrolytic enzymes which remove sialic acid residues from glycoproteins and glycolipids. It is localized in the plasma membrane, and its activity is specific for gangliosides. It may play a role in modulating the ganglioside content of the lipid bilayer.[2]
Interactions
NEU3 has been shown to interact with Grb2.[3]
References
- ^ Wada T, Yoshikawa Y, Tokuyama S, Kuwabara M, Akita H, Miyagi T (Aug 1999). "Cloning, expression, and chromosomal mapping of a human ganglioside sialidase". Biochem Biophys Res Commun 261 (1): 21–7. doi:10.1006/bbrc.1999.0973. PMID 10405317.
- ^ a b "Entrez Gene: NEU3 sialidase 3 (membrane sialidase)". http://www.ncbi.nlm.nih.gov/sites/entrez?Db=gene&Cmd=ShowDetailView&TermToSearch=10825.
- ^ Sasaki, Akinori; Hata Keiko, Suzuki Susumu, Sawada Masashi, Wada Tadashi, Yamaguchi Kazunori, Obinata Masuo, Tateno Hiroo, Suzuki Hiroshi, Miyagi Taeko (Jul. 2003). "Overexpression of plasma membrane-associated sialidase attenuates insulin signaling in transgenic mice". J. Biol. Chem. (United States) 278 (30): 27896–902. doi:10.1074/jbc.M212200200. ISSN 0021-9258. PMID 12730204.
Further reading
- Hu H, Shioda T, Moriya C et al. (1996). "Infectivities of human and other primate lentiviruses are activated by desialylation of the virion surface". J. Virol. 70 (11): 7462–70. PMC 190813. PMID 8892864. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=190813.
- Monti E, Bassi MT, Papini N et al. (2001). "Identification and expression of NEU3, a novel human sialidase associated to the plasma membrane". Biochem. J. 349 (Pt 1): 343–51. doi:10.1042/0264-6021:3490343. PMC 1221155. PMID 10861246. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1221155.
- Wang Y, Yamaguchi K, Wada T et al. (2002). "A close association of the ganglioside-specific sialidase Neu3 with caveolin in membrane microdomains". J. Biol. Chem. 277 (29): 26252–9. doi:10.1074/jbc.M110515200. PMID 12011038.
- Kakugawa Y, Wada T, Yamaguchi K et al. (2002). "Up-regulation of plasma membrane-associated ganglioside sialidase (Neu3) in human colon cancer and its involvement in apoptosis suppression". Proc. Natl. Acad. Sci. U.S.A. 99 (16): 10718–23. doi:10.1073/pnas.152597199. PMC 125023. PMID 12149448. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=125023.
- Hart ML, Saifuddin M, Uemura K et al. (2003). "High mannose glycans and sialic acid on gp120 regulate binding of mannose-binding lectin (MBL) to HIV type 1". AIDS Res. Hum. Retroviruses 18 (17): 1311–7. doi:10.1089/088922202320886352. PMID 12487819.
- Oehler C, Kopitz J, Cantz M (2003). "Substrate specificity and inhibitor studies of a membrane-bound ganglioside sialidase isolated from human brain tissue". Biol. Chem. 383 (11): 1735–42. doi:10.1515/BC.2002.194. PMID 12530538.
- Sasaki A, Hata K, Suzuki S et al. (2003). "Overexpression of plasma membrane-associated sialidase attenuates insulin signaling in transgenic mice". J. Biol. Chem. 278 (30): 27896–902. doi:10.1074/jbc.M212200200. PMID 12730204.
- Ota T, Suzuki Y, Nishikawa T et al. (2004). "Complete sequencing and characterization of 21,243 full-length human cDNAs". Nat. Genet. 36 (1): 40–5. doi:10.1038/ng1285. PMID 14702039.
- Brandenberger R, Wei H, Zhang S et al. (2005). "Transcriptome characterization elucidates signaling networks that control human ES cell growth and differentiation". Nat. Biotechnol. 22 (6): 707–16. doi:10.1038/nbt971. PMID 15146197.
- Ha KT, Lee YC, Cho SH et al. (2004). "Molecular characterization of membrane type and ganglioside-specific sialidase (Neu3) expressed in E. coli". Mol. Cells 17 (2): 267–73. PMID 15179041.
- Stamatos NM, Liang F, Nan X et al. (2005). "Differential expression of endogenous sialidases of human monocytes during cellular differentiation into macrophages". FEBS J. 272 (10): 2545–56. doi:10.1111/j.1742-4658.2005.04679.x. PMID 15885103.
- Kato K, Shiga K, Yamaguchi K et al. (2006). "Plasma-membrane-associated sialidase (NEU3) differentially regulates integrin-mediated cell proliferation through laminin- and fibronectin-derived signalling". Biochem. J. 394 (Pt 3): 647–56. doi:10.1042/BJ20050737. PMC 1383714. PMID 16241905. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1383714.
- Kimura K, Wakamatsu A, Suzuki Y et al. (2006). "Diversification of transcriptional modulation: Large-scale identification and characterization of putative alternative promoters of human genes". Genome Res. 16 (1): 55–65. doi:10.1101/gr.4039406. PMC 1356129. PMID 16344560. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1356129.
- Ueno S, Saito S, Wada T et al. (2006). "Plasma membrane-associated sialidase is up-regulated in renal cell carcinoma and promotes interleukin-6-induced apoptosis suppression and cell motility". J. Biol. Chem. 281 (12): 7756–64. doi:10.1074/jbc.M509668200. PMID 16428383.
- Yamaguchi K, Hata K, Wada T et al. (2006). "Epidermal growth factor-induced mobilization of a ganglioside-specific sialidase (NEU3) to membrane ruffles". Biochem. Biophys. Res. Commun. 346 (2): 484–90. doi:10.1016/j.bbrc.2006.05.136. PMID 16765317.
- Nan X, Carubelli I, Stamatos NM (2007). "Sialidase expression in activated human T lymphocytes influences production of IFN-gamma". J. Leukoc. Biol. 81 (1): 284–96. doi:10.1189/jlb.1105692. PMID 17028199.
- Yoshizumi S, Suzuki S, Hirai M et al. (2007). "Increased hepatic expression of ganglioside-specific sialidase, NEU3, improves insulin sensitivity and glucose tolerance in mice". Metab. Clin. Exp. 56 (3): 420–9. doi:10.1016/j.metabol.2006.10.027. PMID 17292733.
- Wada T, Hata K, Yamaguchi K et al. (2007). "A crucial role of plasma membrane-associated sialidase in the survival of human cancer cells". Oncogene 26 (17): 2483–90. doi:10.1038/sj.onc.1210341. PMID 17334392.
- Azuma Y, Sato H, Higai K, Matsumoto K (2007). "Enhanced expression of membrane-associated sialidase Neu3 decreases GD3 and increases GM3 on the surface of Jurkat cells during etoposide-induced apoptosis". Biol. Pharm. Bull. 30 (9): 1680–4. doi:10.1248/bpb.30.1680. PMID 17827720.
Hydrolase: sugar hydrolases (EC 3.2) 3.2.1: Glycoside hydrolases Cellulase · Alpha-glucosidase (Acid, Neutral AB, Neutral C) · Beta-glucosidase (cytosolic) · Debranching enzymeOtherAmylase (Alpha-Amylase) · Chitinase · Lysozyme · Neuraminidase (NEU1, NEU2, NEU3, NEU4, Bacterial neuraminidase, Viral neuraminidase) · Galactosidases (Alpha, Beta) · alpha-Mannosidase · Glucuronidase · Hyaluronidase · Pullulanase · Glucosylceramidase (lysosomal, non-lysosomal) · Galactosylceramidase · Alpha-N-acetylgalactosaminidase (NAGA) · Alpha-N-acetylglucosaminidase · Fucosidase · Hexosaminidase (HEXA, HEXB) · Iduronidase · Maltase-glucoamylase · Heparanase (HPSE2)3.2.2: Hydrolysing
N-Glycosyl compoundsB enzm: 1.1/2/3/4/5/6/7/8/10/11/13/14/15-18, 2.1/2/3/4/5/6/7/8, 2.7.10, 2.7.11-12, 3.1/2/3/4/5/6/7, 3.1.3.48, 3.4.21/22/23/24, 4.1/2/3/4/5/6, 5.1/2/3/4/99, 6.1-3/4/5-6 Categories:- Human proteins
- Chromosome 11 gene stubs
Wikimedia Foundation. 2010.
