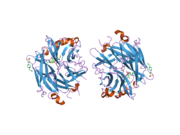- NEU2
-
Sialidase 2 (cytosolic sialidase) 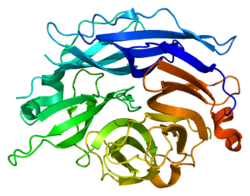
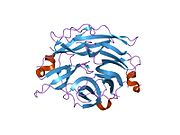
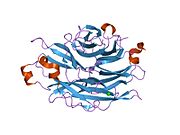
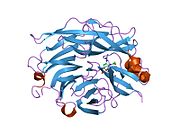
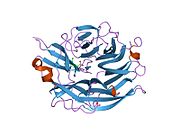
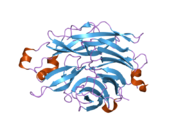
PDB rendering based on 1snt.Available structures PDB 1SNT, 1SO7, 1VCU, 2F0Z, 2F10, 2F11, 2F12, 2F13, 2F24, 2F25, 2F26, 2F27, 2F28, 2F29 Identifiers Symbols NEU2; MGC129579; SIAL2 External IDs OMIM: 605528 MGI: 1344417 HomoloGene: 3927 GeneCards: NEU2 Gene EC number 3.2.1.18 Gene Ontology Molecular function • catalytic activity
• exo-alpha-sialidase activity
• hydrolase activity, acting on glycosyl bondsCellular component • cytoplasm
• cytosolBiological process • metabolic process
• positive regulation of myotube differentiation
• response to ethanol
• positive regulation of myoblast differentiationSources: Amigo / QuickGO RNA expression pattern 
More reference expression data Orthologs Species Human Mouse Entrez 4759 23956 Ensembl ENSG00000115488 ENSMUSG00000079434 UniProt Q9Y3R4 n/a RefSeq (mRNA) NM_005383 NM_015750 RefSeq (protein) NP_005374 NP_056565 Location (UCSC) Chr 2:
233.9 – 233.9 MbChr 1:
89.41 – 89.49 MbPubMed search [1] [2] Sialidase-2 is an enzyme that in humans is encoded by the NEU2 gene.[1][2][3]
This gene belongs to a family of glycohydrolytic enzymes which remove sialic acid residues from glycoproteins and glycolipids. Expression studies in COS7 cells confirmed that this gene encodes a functional sialidase. Its cytosolic localization was demonstrated by cell fractionation experiments.[3]
References
- ^ Monti E, Preti A, Rossi E, Ballabio A, Borsani G (Jun 1999). "Cloning and characterization of NEU2, a human gene homologous to rodent soluble sialidases". Genomics 57 (1): 137–143. doi:10.1006/geno.1999.5749. PMID 10191093.
- ^ Tringali C, Papini N, Fusi P, Croci G, Borsani G, Preti A, Tortora P, Tettamanti G, Venerando B, Monti E (Jan 2004). "Properties of recombinant human cytosolic sialidase HsNEU2. The enzyme hydrolyzes monomerically dispersed GM1 ganglioside molecules". J Biol Chem 279 (5): 3169–3179. doi:10.1074/jbc.M308381200. PMID 14613940.
- ^ a b "Entrez Gene: NEU2 sialidase 2 (cytosolic sialidase)". http://www.ncbi.nlm.nih.gov/sites/entrez?Db=gene&Cmd=ShowDetailView&TermToSearch=4759.
Further reading
- Li CY, Yu Q, Ye ZQ et al. (2007). "A nonsynonymous SNP in human cytosolic sialidase in a small Asian population results in reduced enzyme activity: potential link with severe adverse reactions to oseltamivir". Cell Res. 17 (4): 357–362. doi:10.1038/cr.2007.27. PMID 17426694.
- Chavas LM, Tringali C, Fusi P et al. (2005). "Crystal structure of the human cytosolic sialidase Neu2. Evidence for the dynamic nature of substrate recognition". J. Biol. Chem. 280 (1): 469–75. doi:10.1074/jbc.M411506200. PMID 15501818.
- Gerhard DS, Wagner L, Feingold EA et al. (2004). "The status, quality, and expansion of the NIH full-length cDNA project: the Mammalian Gene Collection (MGC)". Genome Res. 14 (10B): 2121–2127. doi:10.1101/gr.2596504. PMC 528928. PMID 15489334. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=528928.
- Hart ML, Saifuddin M, Uemura K et al. (2003). "High mannose glycans and sialic acid on gp120 regulate binding of mannose-binding lectin (MBL) to HIV type 1". AIDS Res. Hum. Retroviruses 18 (17): 1311–1317. doi:10.1089/088922202320886352. PMID 12487819.
- Strausberg RL, Feingold EA, Grouse LH et al. (2003). "Generation and initial analysis of more than 15,000 full-length human and mouse cDNA sequences". Proc. Natl. Acad. Sci. U.S.A. 99 (26): 16899–16903. doi:10.1073/pnas.242603899. PMC 139241. PMID 12477932. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=139241.
- Bassi MT, Sperandeo MP, Incerti B et al. (2000). "SLC7A8, a gene mapping within the lysinuric protein intolerance critical region, encodes a new member of the glycoprotein-associated amino acid transporter family". Genomics 62 (2): 297–303. doi:10.1006/geno.1999.5978. PMID 10610726.
- Monti E, Preti A, Nesti C et al. (2000). "Expression of a novel human sialidase encoded by the NEU2 gene". Glycobiology 9 (12): 1313–1321. doi:10.1093/glycob/9.12.1313. PMID 10561456.
- Hu H, Shioda T, Moriya C et al. (1996). "Infectivities of human and other primate lentiviruses are activated by desialylation of the virion surface". J. Virol. 70 (11): 7462–70. PMC 190813. PMID 8892864. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=190813.
- Miyagi T, Konno K, Emori Y et al. (1994). "Molecular cloning and expression of cDNA encoding rat skeletal muscle cytosolic sialidase". J. Biol. Chem. 268 (35): 26435–40. PMID 8253770.
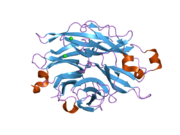
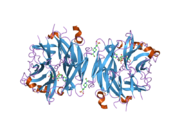
PDB gallery 1snt: Structure of the human cytosolic sialidase Neu21so7: Maltose-induced structure of the human cytolsolic sialidase Neu21vcu: Structure of the human cytosolic sialidase Neu2 in complex with the inhibitor DANA2f0z: Crystal Structure of the Human Sialidase Neu2 in Complex with Zanamivir inhibitor2f10: Crystal Structure of the Human Sialidase Neu2 in Complex with Peramivir inhibitor2f11: Crystal Structure of the Human Sialidase Neu2 in Complex with isobutyl ether mimetic Inhibitor2f12: Crystal Structure of the Human Sialidase Neu2 in Complex with 3- hydroxypropyl ether mimetic Inhibitor2f13: Crystal Structure of the Human Sialidase Neu2 in Complex with 2',3'- dihydroxypropyl ether mimetic Inhibitor2f24: Crystal Structure of the Human Sialidase Neu2 E111Q Mutant2f25: Crystal Structure of the Human Sialidase Neu2 E111Q Mutant in Complex with DANA Inhibitor2f26: Crystal Structure of the Human Sialidase Neu2 E111Q-Q112E Double Mutant2f27: Crystal Structure of the Human Sialidase Neu2 E111Q-Q112E Double Mutant in Complex with DANA Inhibitor2f28: Crystal Structure of the Human Sialidase Neu2 Q116E Mutant2f29: Crystal Structure of the Human Sialidase Neu2 Q116E Mutant in Complex with DANA InhibitorHydrolase: sugar hydrolases (EC 3.2) 3.2.1: Glycoside hydrolases Cellulase · Alpha-glucosidase (Acid, Neutral AB, Neutral C) · Beta-glucosidase (cytosolic) · Debranching enzymeOtherAmylase (Alpha-Amylase) · Chitinase · Lysozyme · Neuraminidase (NEU1, NEU2, NEU3, NEU4, Bacterial neuraminidase, Viral neuraminidase) · Galactosidases (Alpha, Beta) · alpha-Mannosidase · Glucuronidase · Hyaluronidase · Pullulanase · Glucosylceramidase (lysosomal, non-lysosomal) · Galactosylceramidase · Alpha-N-acetylgalactosaminidase (NAGA) · Alpha-N-acetylglucosaminidase · Fucosidase · Hexosaminidase (HEXA, HEXB) · Iduronidase · Maltase-glucoamylase · Heparanase (HPSE2)3.2.2: Hydrolysing
N-Glycosyl compoundsB enzm: 1.1/2/3/4/5/6/7/8/10/11/13/14/15-18, 2.1/2/3/4/5/6/7/8, 2.7.10, 2.7.11-12, 3.1/2/3/4/5/6/7, 3.1.3.48, 3.4.21/22/23/24, 4.1/2/3/4/5/6, 5.1/2/3/4/99, 6.1-3/4/5-6 Categories:- Human proteins
- Chromosome 2 gene stubs
Wikimedia Foundation. 2010.