- Isochoric process
-
An isochoric process, also called a constant-volume process, an isovolumetric process, or an isometric process, is a thermodynamic process during which the volume of the closed system undergoing such a process remains constant. An isochoric process is exemplified by the heating or the cooling of the contents of a sealed, inelastic container: The thermodynamic process is the addition or removal of heat; the isolation of the contents of the container establishes the closed system; and the inability of the container to deform imposes the constant-volume condition.
Contents
Formalism
An isochoric thermodynamic process is characterized by constant volume, i.e. ΔV = 0. The process does no pressure-volume work, since such work is defined by
- ΔW = PΔV,
where P is pressure. The sign convention is such that positive work is performed by the system on the environment.
For a reversible process, the first law of thermodynamics gives the change in the system's internal energy:
dU = dQ − dW
Replacing work with a change in volume gives
dU = dQ − PdV
Since the process is isochoric, dV = 0, the previous equation now gives
dU = dQ
Using the definition of specific heat capacity at constant volume,
Cv = dU / dT,
dQ = nCvdT
Integrating both sides yields

Where C is the specific heat capacity at constant volume, a is initial temperature and b is final temperature. We conclude with:

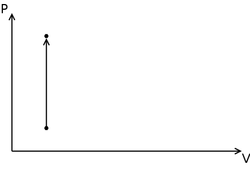 Isochoric Process in the Pressure volume diagram. In this diagram, pressure increases, but volume remains constant.
Isochoric Process in the Pressure volume diagram. In this diagram, pressure increases, but volume remains constant.
On a pressure volume diagram, an isochoric process appears as a straight vertical line. Its thermodynamic conjugate, an isobaric process would appear as a straight horizontal line.
Ideal gas
If an ideal gas is used in an isochoric process, and the quantity of gas stays constant, then the increase in energy is proportional to an increase in temperature and pressure. Take for example a gas heated in a rigid container: the pressure and temperature of the gas will increase, but the volume will remain the same.
Ideal Otto cycle
The ideal Otto cycle is an example of an isochoric process when it is assumed that the burning of the gasoline-air mixture in an internal combustion engine car is instantaneous. There is an increase in the temperature and the pressure of the gas inside the cylinder while the volume remains the same.
Etymology
The noun isochor and the adjective isochoric are derived from the Greek words ἴσος (isos) meaning "equal", and χώρα (chora) meaning "space."
See also
- Isobaric process
- Adiabatic process
- Cyclic process
- Isothermal process
- Polytropic process
References
External links
Categories:- Thermodynamic processes
Wikimedia Foundation. 2010.





