- Clausius theorem
-
The Clausius theorem (1855) states that in a cyclic process
The equality holds in the reversible case[1] and the '<' is in the irreversible case. The reversible case is used to introduce the state function entropy. This is because in cyclic process the variation of a state function is zero.
Contents
Proof
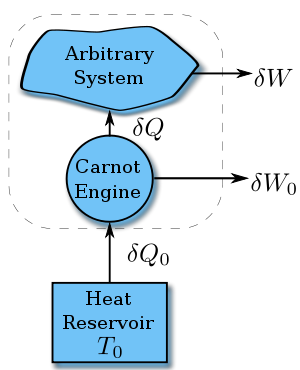
Suppose a system absorbs heat δQ at temperature T. Since the value of
 does not depend on the details of how the heat is transferred, we can assume it is from a Carnot engine, which in turn absorbs heat δQ0 from a heat reservoir with constant temperature T0.
does not depend on the details of how the heat is transferred, we can assume it is from a Carnot engine, which in turn absorbs heat δQ0 from a heat reservoir with constant temperature T0.According to the nature of Carnot cycle,
Therefore in one cycle, the total heat absorbed from the reservoir is
Since after a cycle, the system and the Carnot engine as a whole return to its initial status, the difference of the internal energy is zero. Thus according to First Law of Thermodynamics,
- Q0 = ΔU + W + W0 = W + W0 = Wtotal
According to the Kelvin statement of Second Law of thermodynamics, we cannot drain heat from one reservoir and convert them entirely into work without making any other changes, so
Combine all the above and we get Clausius inequality
If the system is reversible, then reverse its path and do the experiment again we can get
Thus
See also
References
External links
- Judith McGovern (2004-03-17). "Proof of Clausius's theorem". http://theory.ph.man.ac.uk/~judith/stat_therm/node30.html. Retrieved Octorber 4,2010.
- "The Clausius Inequality And The Mathematical Statement Of The Second Law". http://ronispc.chem.mcgill.ca/ronis/chem213/hnd10.pdf. Retrieved October 5, 2010.
Categories:- Thermodynamics
- Physics theorems
Wikimedia Foundation. 2010.