- Antimony trioxide
-
Antimony trioxide  Antimony(III) oxideOther names
Antimony(III) oxideOther namesIdentifiers CAS number 1309-64-4 
ChemSpider 25727 
UNII P217481X5E 
KEGG C19192 
RTECS number CC5650000 Jmol-3D images Image 1 - O=[Sb]O[Sb]=O
Properties Molecular formula Sb2O3 Molar mass 291.52 g/mol Appearance white solid Density 5.2 g/cm3, α-form Melting point 656 °C
Boiling point 1425 °C (sublimes)
Solubility in water 1.21 mg/100 mL (0 °C)
1.80 mg/100 mL (20 °C)
8.20 mg/100 mL (100 °C), with hydrolysisSolubility insoluble Refractive index (nD) 2.087 Structure Crystal structure cubic (α)<570 °C
orthorhombic (β) >570 °CCoordination
geometrypyramidal Dipole moment zero Hazards MSDS External MSDS EU classification Harmful (Xn)
Carc. Cat. 3R-phrases R40 S-phrases (S2), S22, S36/37 NFPA 704 LD50 7000 mg/kg, oral (rat) Related compounds Other anions Antimony trisulfide Other cations Arsenic trioxide
Bismuth trioxideRelated compounds Diantimony tetraoxide
Antimony pentoxideSupplementary data page Structure and
propertiesn, εr, etc. Thermodynamic
dataPhase behaviour
Solid, liquid, gasSpectral data UV, IR, NMR, MS  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Antimony trioxide is the inorganic compound with the formula Sb2O3. It is the most important commercial compound of antimony. It is found in nature as the minerals valentinite and senarmontite.[1] Like most polymeric oxides, Sb2O3 dissolves in aqueous solutions only with hydrolysis.
Contents
Production and properties
Global production of antimony trioxide in 2005 was 120,000 tonnes, an increase from 112,600 tonnes in 2002. China produces the largest share (47 %) followed by US/Mexico (22 %), Europe (17 %), Japan (10 %) and South Africa (2 %) and other countries (2%).[2]
Antimony trioxide is mainly produced via the smelting of stibnite ore, which is oxidised to crude Sb2O3 using furnaces operating at approximately 850 to 1,000 °C. The transformation is described as follows:
- 2 Sb2S3 + 9 O2 → 2 Sb2O3 + 6 SO2
Crude Sb2O3 is purified by sublimation, which allows it to be separated from the more volatile arsenic trioxide. This step is relevant because antimony ores commonly contain significant amounts of arsenic.
Antimony oxide is also obtained via antimony trichloride, which can be obtained from stibnite.
- 2 Sb2S3 + 3 CaCl2 + 6 O2 → 4 SbCl3 + 3 CaSO4
After fractional distillation to separate it from arsenic trichloride, SbCl3 can be hydrolyzed to the oxide:
- 2 SbCl3 + 3 H2O → Sb2O3 + 6 HCl
Intermediates in the hydrolysis include the oxychlorides SbOCl and Sb4O5Cl2.
Although impractical for commercial purposes, Sb2O3 can be prepared by burning elemental antimony in air:
- 4 Sb + 3 O2 → 2 Sb2O3
Properties
Antimony trioxide is an amphoteric oxide, dissolving in alkaline solution to give antimonites and in acid solution to given a range of polyantimonous acids. It can be readily oxidized to antimony pentoxide and related antimony(V) compounds, but it is also easily reduced to antimony, sometimes with production of stibine.
Structure
The structure of Sb2O3 depends on the temperature of the sample. Dimeric Sb4O6 is the high temperature (1560 °C) gas.[3] Sb4O6 molecules are bicyclic cages, similar to the related oxide of phosphorus(III), phosphorus trioxide.[4] The cage structure is retained in a solid that crystallizes in a cubic habit. The Sb-O distance is 197.7 pm and the O-Sb-O angle of 95.6°.[5] This form exists in nature as the mineral senarmontite.[4] Below 606 °C, the more stable form is orthorhombic, consisting of pairs -Sb-O-Sb-O- chains that are linked by oxide bridges between the Sb centers. This form exists in nature as the mineral valentinite.[4]
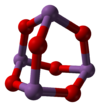

Sb4O6 senarmontite valentinite Uses
The annual consumption of antimony trioxide in the United States and Europe is approximately 10,000 and 25,000 tonnes, respectively. The main application is for flame retardants in combination with halogenated materials. The combination of the halides and the antimony being key to the flame-retardant action for polymers, helping to form less flammable chars. Such flame retardants are found in electrical apparatus, textiles, leather, and coatings.[6]
Other applications:
- Antimony trioxide is an opacifying agent for glasses, ceramics and enamels.
- Some specialty pigments contain antimony.
- Antimony trioxide is a useful catalyst in the production of polyethylene terephthalate (PET plastic) and the vulcanization of rubber.
- Flame retardant for textiles, leather, polymers, and coatings.
Safety
The toxicity of Sb2O3 is topical because it is a likely byproduct of the combustion of some materials "fireproofed" with antimony compounds. The oxides of arsenic, antimony, and bismuth are comparable in their toxicity, but their volatilities differ widely. Antimony trioxide has suspected carcinogenic potential for humans.[6] Its TLV is 0.5 mg/m3, as for most antimony compounds.[7]
References
- ^ Greenwood, N. N.; & Earnshaw, A. (1997). Chemistry of the Elements (2nd Edn.), Oxford:Butterworth-Heinemann. ISBN 0-7506-3365-4.
- ^ http://ecb.jrc.ec.europa.eu/DOCUMENTS/Existing-Chemicals/RISK_ASSESSMENT/REPORT/datreport415.pdf Risk Assessment of Diantimony Trioxide
- ^ Egon Wiberg, Arnold Frederick Holleman (2001) Inorganic Chemistry, Elsevier ISBN 0123526515
- ^ a b c Wells, A.F. (1984) Structural Inorganic Chemistry, Oxford: Clarendon Press. ISBN 0-19-855370-6.
- ^ C. Svensson “Refinement of the crystal structure of cubic antimony trioxide, Sb2O3” Acta Crystographica, 1975, volume B31, pp. 2016-2018. doi:10.1107/S0567740875006759
- ^ a b Sabina C. Grund, Kunibert Hanusch, Hans J. Breunig, Hans Uwe Wolf “Antimony and Antimony Compounds” in Ullmann's Encyclopedia of Industrial Chemistry 2006, Wiley-VCH, Weinheim. doi: 10.1002/14356007.a03_055.pub2
- ^ Inhalation Developmental Toxicity Studies In Rats With Antimony Trioxide (Sb2O3). Newton PE; Schroeder RE; Zwick L; Serex T; Toxicologist 2004 Mar;78(1-S):38
Further reading
- Institut national de recherche et de sécurité (INRS), Fiche toxicologique nº 198 : Trioxyde de diantimoine, 1992.
- The Oxide Handbook, G.V. Samsonov, 1981, 2nd ed. IFI/Plenum, ISBN 0-306-65177-7
External links
- International Chemical Safety Card 0012
- ESIS: European chemical Substances Information System
- Antimony Market And Price
- Société industrielle et chimique de l'Aisne
Antimony compounds Categories:- Antimony compounds
- Oxides
- Inorganic pigments
- Common oxide glass components
- IARC Group 2B carcinogens
- Sesquioxides
- Cubic minerals
Wikimedia Foundation. 2010.

